-
BHF awards £35m funding to nine UK universities for cardiovascular disease
- Source: drugdu
- 120
- June 1, 2024
-
Multinational companies target the trillion-dollar medical equipment “trade-in” market
- Source: drugdu
- 81
- June 1, 2024
-
With M&A Deal, Click Therapeutics’ Digital Prospects in Metabolic Disease Get Better
- Source: drugdu
- 73
- May 29, 2024
-
New AI Tool Classifies Brain Tumors More Quickly and Accurately
- Source: drugdu
- 74
- May 22, 2024
-
MHRA’s AI Airlock to address challenges for regulating AI medical devices
- Source: drugdu
- 117
- May 15, 2024
-
Oregon and Lantern collaborate to develop drug candidate for various cancer indications
- Source: drugdu
- 100
- May 13, 2024
-
BigHat Biosciences partners with Janssen Biotech
- Source: drugdu
- 109
- May 5, 2024
-
UK sets out position on regulating AI as a medical device
- Source: drugdu
- 112
- May 5, 2024
-
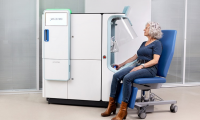
Robotic Blood Drawing Device to Revolutionize Sample Collection for Diagnostic Testing
- Source: https://www.labmedica.com/pathology/articles/294801059/robotic-blood-drawing-device-to-revolutionize-sample-collection-for-diagnostic-testing.html
- 152
- May 4, 2024
-
DP Technology DevDay 2024 Showcases Large Science Models and Announces Open Science Initiative
- Source: https://en.prnasia.com/releases/global/dp-technology-devday-2024-showcases-large-science-models-and-announces-open-science-initiative-445188.shtml
- 108
- May 3, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.