Robotic Blood Drawing Device to Revolutionize Sample Collection for Diagnostic Testing
May 4, 2024
Source: https://www.labmedica.com/pathology/articles/294801059/robotic-blood-drawing-device-to-revolutionize-sample-collection-for-diagnostic-testing.html
 846
846
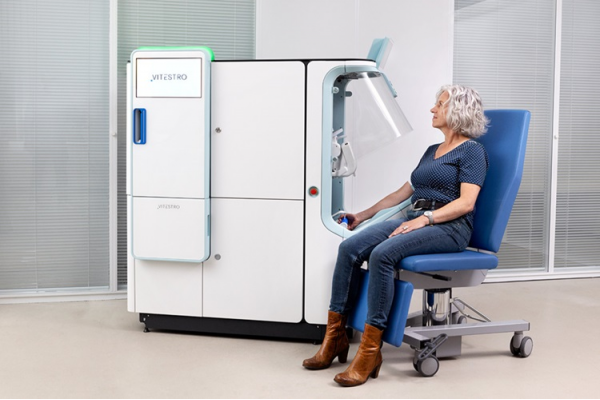
Blood drawing is performed billions of times each year worldwide, playing a critical role in diagnostic procedures. Despite its importance, clinical laboratories are dealing with significant staff shortages, which impact their ability to deliver timely test results and maintain satisfactory patient care. Now, an innovative robotic blood drawing device for the medical laboratory market could help ease staff workload and provide a more consistent patient experience.
Developed by Vitestro (Utrecht, The Netherlands), this innovative blood-drawing device is designed to perform safe and accurate blood draws. It utilizes artificial intelligence (AI) for ultrasound-guided 3D reconstruction and ensures submillimeter precision in needle insertion. This high level of accuracy and consistency in blood collection is achieved through a combination of AI, advanced imaging technologies, and robotics. By automating blood draws, Vitestro’s device not only reduces the physical demand on staff but also enhances the satisfaction of both patients and healthcare providers. The introduction of this device marks a major innovation in blood collection, addressing the acute shortage of healthcare personnel and significantly improving the efficiency of clinical laboratories.
This innovation in phlebotomy offers a complementary approach to traditional manual blood sample collection. In the near future, patients can opt for autonomous blood draws using the device under the supervision of a trained healthcare professional. Vitestro has initiated the A.D.O.P.T. Trial to evaluate its autonomous blood drawing device on a large scale, planning to involve over 10,000 patients. This two-year study aims to further develop the device’s capabilities and obtain the required performance and safety data for regulatory approvals. Following the trial, Vitestro anticipates obtaining CE marking to enable autonomous venipuncture by the end of 2024. Several units of the device have already been pre-ordered and are expected to be installed in numerous European hospitals by the end of this year, signifying a significant step forward in automating the blood drawing process and enhancing patient care in clinical settings.
“Our technology represents a major step forward in healthcare innovation, benefitting both the institution and the patient,” said Brian Joseph, commercial director and co-founder at Vitestro. “We are actively expanding operations to grow in other important international markets, particularly within the United States.”
By editor
Read more on
- Gusekirumab Injection Accepted by CDE, Multiple Pipelines Advancing Simultaneously March 4, 2026
- Yifan Pharmaceutical’s teriparatide injection has been accepted by the CDE (Center for Drug Evaluation), adding a new domestic player to the osteoporosis treatment field March 4, 2026
- //news.yaozh.com/archive/47318.html PD-1 sales surge March 4, 2026
- A major breakthrough! Roche’s oral BTK inhibitor achieves its third Phase III clinical trial victory, a game-changer in the multi-billion dollar MS (manufactured pharmaceuticals) market. March 4, 2026
- GB19 Injection Approved for Clinical Trials of Cutaneous Lupus Erythematosus March 4, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



