Essential forms for occupying the Indian pharmaceutical market
August 7, 2018
Source: Ddu
 2,160
2,160

As one of the biggest generic drug manufacturing countries in the world, India’s pharmaceutical industry has increased by 13% - 14% within the last 5 years. As a result, many companies are looking to take advantage of this growth but are in need of guidance. In response to the numerous inquiries we have received here at Drugdu.com, we have listed three essential forms regarding the rules of drug registration in India.
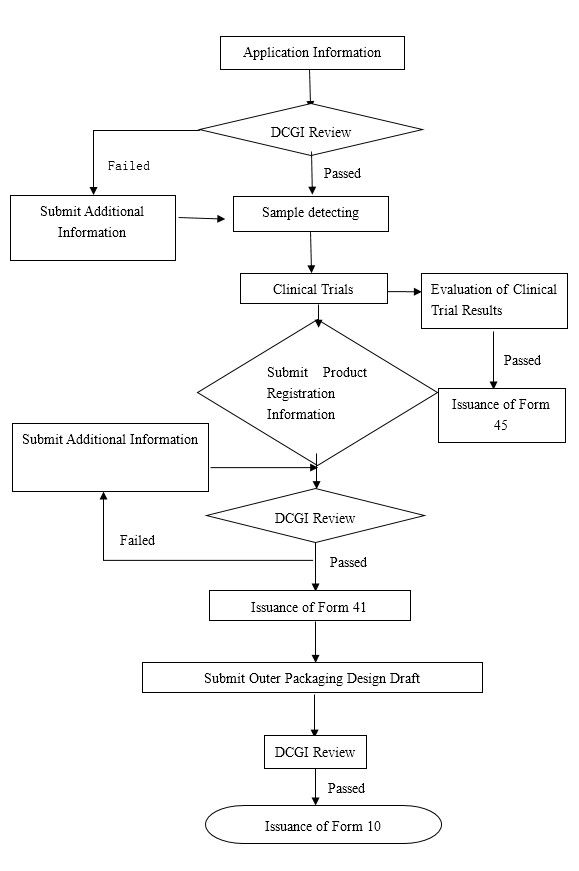
As the first threshold for products to enter the Indian market, Form 45 requires suppliers to provide PMF, SMF, CTD as wells as GMP authentication along with various other registration documents. After the approval by the DCGI (Drug Controller General of India), the next phase includes the sample trials and clinical trials process. Normally the quantity requirements for sample trials is inspection of three continuous batches of the entire quantity of all the products. It is worth noting that all batches of products for sample trials must be brought into correspondence with the batches for clinical trials. Regarding clinical trials, the time period and case requirements for clinical trials in India will be determined by existing clinical trial results or whether there is similar local reference listed drugs under review to appear on the market.
Take stage 3 of clinical trials as an example, the number of participating people will be 300 - 500, and the process usually takes 18 months. If the sample trials are passed and the results of the clinical trials turned out positive, you will soon be issued Form 45, indicating great progress to enter the Indian market. Below is an example of Form 45:

When Form 45 has been finalized, 70% of the entire process will have been completed. It does, however, take approximately 2 years to complete sample and clinical trials.
The next step is applying for Form 41, the Certificate of Registration. The documentation requirements of Form 41 are basically the same as Form 45, hence two copies of GMP and COPP certification and the production license will be necessary. If any explanations or supplementations are required during the verification process, the DCGI will send a letter for information supplementation to the applicant. In the event that there is a shortage regarding the supplementations, the registration could be rejected. The DCGI will make a final assessment according to all the documents received, then issue Form 41.

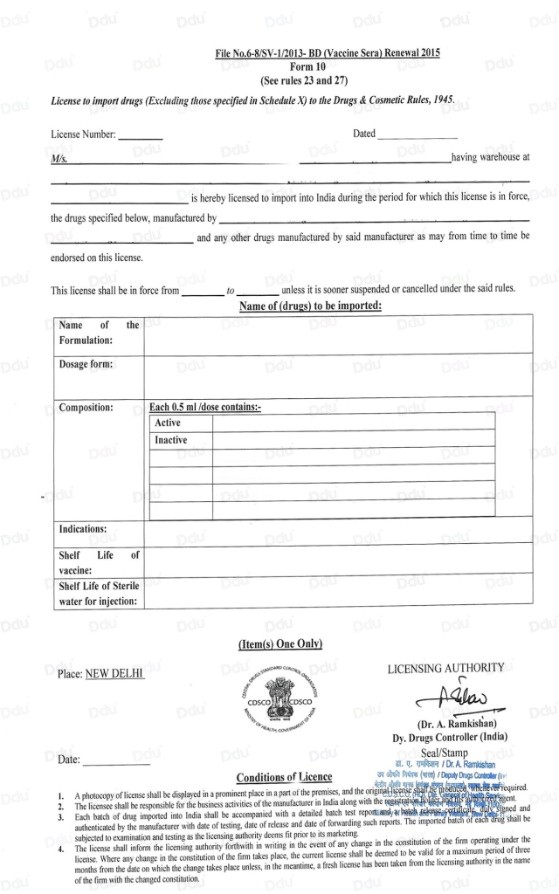
Form 10 is the license to Import Drugs and is one of the most important documents for pharmaceutical imports to the Indian market since the drug importing license number will be listed on this form.
When the application of Form 41 has been finalized, the final version of the English product packaging design draft (including packaging, labels and instructions) also needs to be submitted to the DCGI.
REMINDER: Regarding vaccines, the drug electronic regulatory code from the country of origin needs to be retained, and a small space will be required on the packaging for the Indian importing license number. The DCGI will issue From 10 after all the documents have been approved.
Flowchart of the Indian drug registration process
For your convenience, we have compiled a flow chart of the Indian drug registration process:
By Ddu
Read more on
- 【EXPERT Q&A】Is it mandatory to conduct clinical trials for drug registration in India? August 9, 2023
- Important Registration Regulations for Exporting Health Care Products to Vietnam March 31, 2022
- Halt in E-Cigarette Sales by Indian Health Ministry August 30, 2018
- WHO Reports a Doctor Being a New Probable Case of Ebola in Congo’s Violence Struck Area August 28, 2018
- Restart of Measles Vaccination can Prevent an Outbreak August 28, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



