Siemens gets CE-Mark for MR scanner Magnetom Terra
August 24, 2017
Source: medicaldevice
 840
840
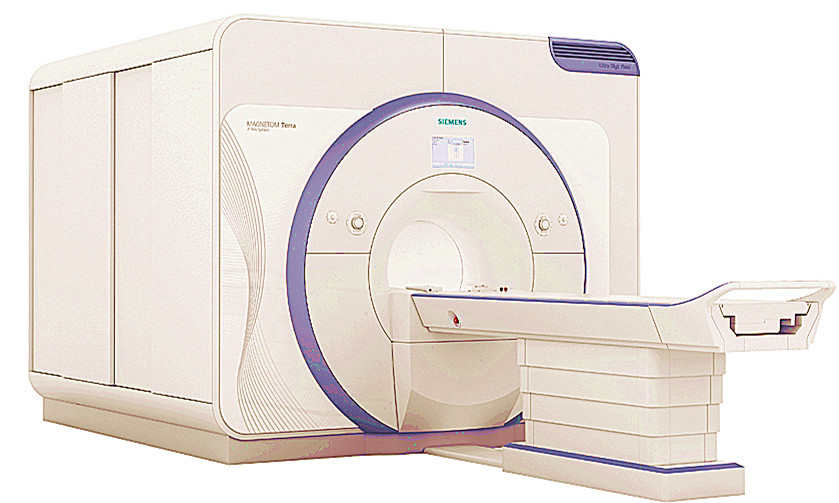
Siemens Healthineers has received CE-Mark for its ultra-high-field 7T magnetic resonance (MR) scanner Magnetom Terra, making it available for neurological and musculoskeletal clinical examinations in Europe.
In addition to detailed insights of the musculoskeletal system, Magnetom Terra’s high spatial and spectral resolution enables imaging of metabolic processes in the brain, as well as visualisation of neurological diseases such as Alzheimer's, epilepsy, and multiple sclerosis (MS).
It is expected that the high-resolution of the scanner will provide clear pictures of brain lesions, including better white and gray matter differentiation in epilepsy and improved visibility in gray matter in multiple sclerosis (MS).
Siemens Healthineers Magnetic Resonance Imaging senior vice-president and general manager Christoph Zindel said: “Having now gained approval for clinical use, we are expanding the scope of diagnostic MRI 15 years after 3T scanners first became established.
"With this new clinical field strength, we can achieve a new level of detail in anatomy and function, helping further pave the way for precision medicine."
“With this new clinical field strength, we can achieve a new level of detail in anatomy and function, helping further pave the way for precision medicine.”
The dual mode functionality of Magnetom Terra facilitates an easy switch between the clinical protocols and new research methods, making it ideal for translational research.
Use of the new MR scanner will enable anatomical imaging of small pathologies and sub-cortical brain activation, while it will also aid the assessment of anatomy, function and metabolism of body tissue.
The scanner uses an actively shielded whole-body magnet that is manufactured at Siemens Magnet Technology in Oxford, UK, and is 50% lighter than previous magnets.
By DduRead more on
- Discovery of Rosehip Neurons in Humans August 29, 2018
- Celgene Collaboration with Skyhawk for Drug Discovery against Neurological Diseases June 28, 2018
- mHealth app for clinical decision support improves diagnosis time, test ordering April 27, 2018
- GE Healthcare and Roche join forces to create clinical decision support tools January 12, 2018
- Researchers find potential path to repair multiple sclerosis-damaged nerves December 27, 2017
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



