Experimental Ebola Treatments Approved by Congo as it Sees Flare-Up in Cases
August 24, 2018
Source: Reuters
 1,465
1,465
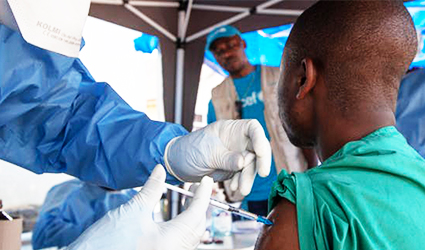
Four additional untried treatment solutions for the Ebola virus danger have received a green signal from the Democratic Republic of Congo as a move from the health ministry in its efforts to control the virus’ spread.
In case of an existing outbreak, in a foremost attempt, the U.S.-originated mAb114 treatment was initiated to be given to Ebola sufferers by healthcare professionals last week.
A “positive evolution” was observed in patients undergoing the mAb114 treatment since August 11 as remarked by the health ministry in a bulletin. However, the virus has not been contained.
Included in the four extra treatments sanctioned by the ethics heads of Congo Republic are Israel’s Gilead Sciences developed Remdesivir; San Diego’s Mapp Pharmaceutical’s parenteral infusion ZMapp; Japanese medicine Favipiravir; and the Regn3450 – 3471 – 3479.
The health ministry bulletin said that the town of Beni which is reportedly performing favorably, saw the first patient to be given Remdesivir. Latest figures suggest that Ebola has taken four lives and diseased six more. The total mortality count equals to 59 and verified cases account to 75 since the last month.
Greater than double compared to any other nation, Congo has suffered not less than ten Ebola virus breakouts post its discovery in north Congo in 1976.
Read more on
- Ebola ‘no longer incurable’ August 15, 2019
- WHO Reports a Doctor Being a New Probable Case of Ebola in Congo’s Violence Struck Area August 28, 2018
- Breakthrough Device Designation Granted to Digital Intervention for Alzheimer’s August 27, 2018
- FDA Approved Eye Drop Oxervate to Treat Neurotrophic Keratitis August 27, 2018
- First Dual-Lead Nerve Stimulator for Pain Management gets FDA Nod August 22, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



