Camber spine wins FDA nod for Spira interbody fusion device
August 17, 2017
Source: massdevice
 888
888
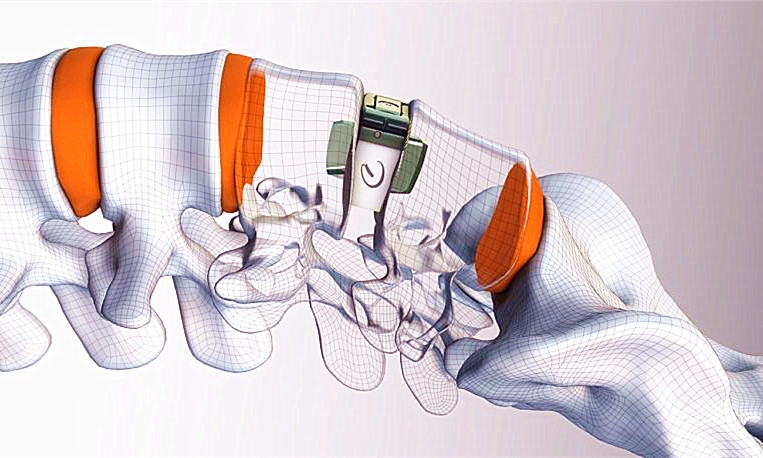
Camber Spine Technologies' Spira open matrix anterior lumbar interbody fusion device won FDA clearance from the FDA.
The Wayne, Penn.-based company said the Spira device consists of spiral support arches designed to increase fusion rates and stabilization. The company said the spiral support arches also decrease subsidence by load sharing over the entire endplate while also maximizing bone graft capacity.
“Camber Spine is very excited to be launching our first in a series of spinal implants using 3D printed – additive manufacturing. This specialized manufacturing technology allows us to create these truly unique patented structures featuring open arched matrices and proprietary surfaces designed to enhance fusion and promote bone growth. In the coming months we will be launching a series of five Spira spinal interbody cages for cervical, lateral, and posterior lumbar spine. Extremity implants and custom implants for salvage and complex deformity implants are also under development. We believe that the addition of Spira and Enza MIS Integrated interbody devices to our product portfolio create a foundation of patented implant solutions that will drive the growth of Camber Spine,” CEO Daniel Pontecorvo said in a press release.
The Spira open matrix ALIF is cleared with an indication for skeletally mature patients with degenerative disc disease at 1 or 2 contiguous levels from L2-S1, the company said, and is designed to be used with FDA-cleared supplementary fixation systems.
By DduRead more on
- Breakthrough Device Designation Granted to Digital Intervention for Alzheimer’s August 27, 2018
- FDA Approved Eye Drop Oxervate to Treat Neurotrophic Keratitis August 27, 2018
- Experimental Ebola Treatments Approved by Congo as it Sees Flare-Up in Cases August 24, 2018
- First Dual-Lead Nerve Stimulator for Pain Management gets FDA Nod August 22, 2018
- Bristol-Myers Squibb’s Opdivo Ensures a Lung Cancer Position August 22, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



