The First Drug to Treat Infants Sick With Malaria Is Approved by Swiss Authority
July 10, 2025
Source: drugdu
 141
141
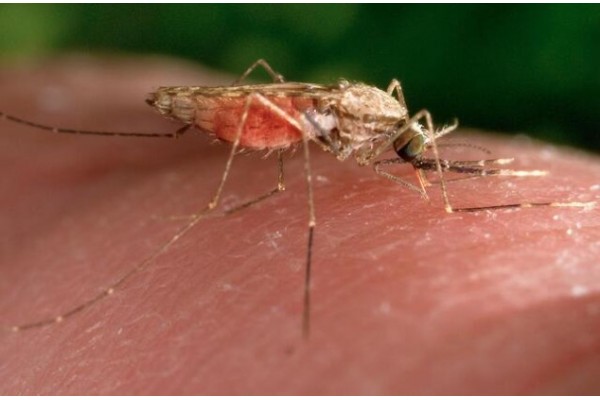 GENEVA (AP) — Switzerland's medical products authority has granted the first approval for a malaria medicine designed for small infants, touted as an advance against a disease that takes hundreds of thousands of lives — nearly all in Africa — each year.
GENEVA (AP) — Switzerland's medical products authority has granted the first approval for a malaria medicine designed for small infants, touted as an advance against a disease that takes hundreds of thousands of lives — nearly all in Africa — each year.
Swissmedic gave a green light Tuesday for the medicine from Basel-based pharmaceutical company Novartis for treatment of babies with body weights between 2 and 5 kilograms (nearly 4½ to 11 pounds), which could pave the way for hard-hit African nations to follow suit in coming months.
The agency said that the decision is significant in part because it's only the third time it has approved a treatment under a fast-track authorization process, in coordination with the World Health Organization, to help developing countries access needed treatment.
The newly approved medication, Coartem Baby, is a combination of two antimalarials. It is a lower dose version of a tablet previously approved for other age groups, including older children.
Dr. Quique Bassat, a malaria expert not affiliated with the Swiss review, said the burden of malaria in very young children is “relatively low” compared to older kids.
But access to such medicines is important to all, he said.
Ruairidh Villar, a Novartis spokesperson, said that eight African countries took part in the assessment and are expected to approve the medicine within 90 days. The company said that it's planning on a rollout on a “largely not-for-profit basis” in countries where malaria is endemic.
Dr. Bhargavi Rao, co-director of the Malaria Centre at the London School of Hygiene and Tropical Medicine, noted that malaria cases continue to rise — especially in crisis-hit countries — despite new vaccines and programs targeting the mosquitoes that spread the parasite.
She said access strategies for the new medicine must include a look at where needs are greatest, and urged clarity on pricing.
“We need transparency around what Novartis’ ‘largely not for profit’ statement means including publicly available pricing, which countries will benefit and how long for,” she wrote in an email.
Still, she said it was “significant to finally have a suitable and safe treatment for very young children — more than 20 years since WHO first pre-qualified Coartem for older age groups.
She noted the announcement comes as resistance to antimalarials has been growing and many traditional donor countries have been sharply cutting outlays for global health — including for malaria programming and research.
The mosquito-borne illness is the deadliest disease in Africa, whose 1.5 billion people accounted for 95% of an estimated 597,000 malaria deaths worldwide in 2023, according to WHO. More than three-quarters of those deaths were among children.
Copyright 2025 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed.
By editorRead more on
- Rovaxitinib approved for marketing, filling the demand for myelofibrosis treatment March 2, 2026
- Warrant Pharmaceuticals’ active pharmaceutical ingredient receives Brazil’s first official GMP certification March 2, 2026
- Merck’s New Story March 2, 2026
- Rongchang Biotechnology has turned a profit! March 2, 2026
- Jiuyuan Gene’s “Simeglucopyranoside” for weight loss (Jikeqin®) has been submitted for market approval March 2, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



