PD-1+LAG3 work together to “break through”
May 29, 2025
Source: drugdu
 272
272
The 2025 ASCO Annual Meeting is about to be held. At present, the regular abstracts have been released to the public, and many domestic new drugs have performed well. Among them, LBL-007 of Weilizhibo has made its debut again with breakthrough data. This is the fifth consecutive year that the drug has been selected for the ASCO meeting.
LBL-007 is a fully human IgG4 monoclonal antibody targeting LAG3. Its clinical progress ranks among the top three in the world (except for the only LAG3-targeted drug on the market). It is also the first antibody of its kind to be proven to be effective against nasopharyngeal carcinoma.
Clinical data show that LBL-007 combined with first-line therapy for NPC patients has an ORR of 83.3% and a median PFS of 15.8 months, which is significantly higher than the existing standard regimen. With its excellent safety and efficacy, LBL-007 is expected to become a new benchmark for first-line treatment of recurrent or metastatic NPC (R/M NPC).
01 Significantly improved efficacy
LAG3 is an immune checkpoint receptor that is activated after binding to a ligand and can promote tumor immune escape by inhibiting T cell function. Studies have shown that the combination of LAG3 inhibitors and PD-1/PD-L1 drugs can enhance the anti-tumor function of T cells, showing a strong synergistic effect in cancer treatment, and providing a new direction for breaking through existing treatment bottlenecks.
Mechanistically, when PD-1 and LAG3 are co-expressed, they can jointly drive T cell exhaustion through different pathways. In a melanoma mouse model, compared with the knockout of PD-1 or LAG3 alone, CD8+ T cells with simultaneous knockout of PD-1 and LAG3 showed stronger tumor killing effects and survival abilities, as well as enrichment of effector-like and interferon-responsive genes, leading to enhanced IFN-γ release. Clinical trials have also shown that combined anti-LAG3 and anti-PD-1 therapy can enhance CD8+ T cell receptor signaling, lower the threshold of TCR activation, change the CD8+ T cell differentiation pattern, and increase cytotoxicity while maintaining exhaustion characteristics. These studies have collectively confirmed that LAG3 and PD-1 can synergistically drive T cell exhaustion, providing a new strategy for combined immunotherapy.
LBL-007 is designed to target a unique epitope of LAG3, bind to LAG3 with high affinity, and block LAG3 from binding to all four recognized immunosuppressive ligands, including MHC-II, LSECtin, Gal-3, and FGL-1. After binding to LAG3, LBL-007 induces effective cellular endocytosis, inhibits LAG3 expression on the cell surface, and thus further blocks ligand interactions and enhances immune responses.
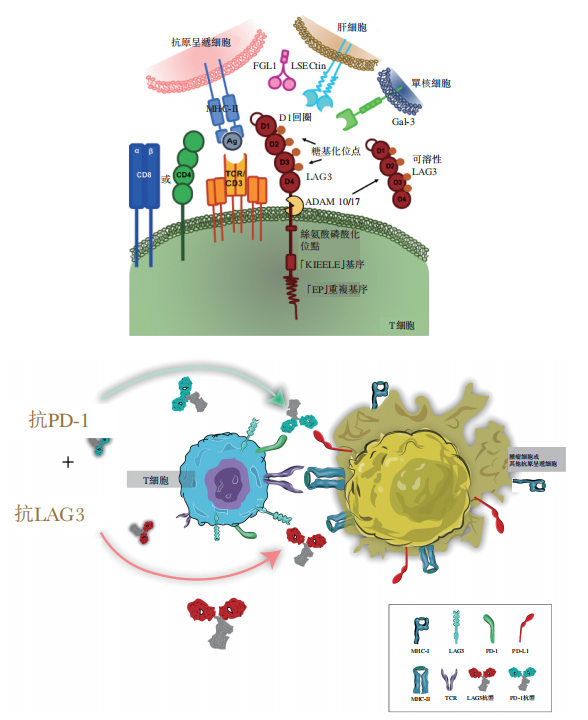
Figure 1. Mechanism of action of LBL-007 and its combination with PD-1 antibodies
Image source: Weilizhibo prospectus
The results of the study showed that the objective response rate (ORR) of patients who received LBL-007, tislelizumab and chemotherapy reached 83.3%, an increase of nearly 20 percentage points compared to 69.5% of patients who only used tislelizumab combined with chemotherapy. This shows that the addition of LBL-007 significantly enhanced the tumor response rate, allowing more patients to benefit from treatment and achieve tumor shrinkage or stabilization.
What is more noteworthy is the significant extension of progression-free survival (PFS). The median PFS of the combined LBL-007 treatment group reached 15.8 months, which is more than 6 months longer than the historical data of 9.6 months for Tiredo plus chemotherapy, an increase of more than 70%. This achievement means that after receiving treatment, patients can maintain stable disease for a longer period of time, delay disease progression, and greatly improve the quality of life and prognosis of patients. For patients with recurrent or metastatic nasopharyngeal carcinoma, this is undoubtedly a huge blessing, winning them more valuable treatment windows.
In addition, although the OS data of LBL-007 is not yet mature, it has shown a trend of benefit and has the potential to surpass the survival benefit of the tislelizumab + GP regimen.
02 A solid backing that can stand the test of time
Safety is another core concern in clinical trials of new drugs.
Previously, the safety of LBL-007 has been verified in multiple clinical studies. Among them, in the Phase Ib/II clinical study of LBL-007 combined with toripalimab in the treatment of advanced solid tumors, the combination therapy showed good safety: the incidence of treatment-related adverse reactions above grade 3 was only 11.3%, the incidence of adverse reactions leading to treatment termination was only 8.8%, no treatment-related deaths occurred, and no unexpected adverse events occurred. Compared with the relatlimab combined with nivolumab regimen (the incidence of patients with grade 3 or 4 treatment-related adverse events was 18.9%), the LBL-007 combination regimen highlights the tolerability advantage.
ASCO 2025 data once again confirmed the good safety of the LBL-007 combination regimen.
The LBL-007 clinical trial announced this time uses a four-drug combination regimen, namely LBL-007 combined with tislelizumab (PD-1 antibody) and gemcitabine and cisplatin (GP chemotherapy regimen). Clinical data showed that compared with the use of tislelizumab combined with chemotherapy alone, no new adverse reaction signals were observed after the addition of LBL-007. The proportion of LBL-007 leading to drug discontinuation was very low (4.8%), even lower than the tislelizumab combined with chemotherapy regimen (5.3%).
Chemotherapy drugs themselves have strong toxic reactions and may cause a series of adverse events. The latest data show that LBL-007 has good compliance and its addition has not changed the overall safety profile. The safety risks faced by patients during treatment still mainly come from chemotherapy drugs, not LBL-007 itself.
This key fact provides a shot in the arm for clinicians and patients.
03 Leading a new era of tumor immunotherapy
Based on the above excellent safety and efficacy data, the relevant team is actively advancing key Phase III clinical trials to further verify the advantages of this combination therapy.
With the advancement of Phase III clinical trials, LBL-007 combined with tislelizumab and chemotherapy is expected to rewrite global treatment guidelines and become a new standard for first-line treatment of recurrent or metastatic nasopharyngeal carcinoma.
Moreover, the breakthrough progress of LBL-007 has also successfully verified the value of the LAG3 target in the field of cancer treatment. Its indications may be expanded to other LAG3-high-expressing tumors (such as non-small cell lung cancer and colorectal cancer), bringing hope to more patients.
https://news.yaozh.com/archive/45540.html
By editorRead more on
- Rovaxitinib approved for marketing, filling the demand for myelofibrosis treatment March 2, 2026
- Warrant Pharmaceuticals’ active pharmaceutical ingredient receives Brazil’s first official GMP certification March 2, 2026
- Merck’s New Story March 2, 2026
- Rongchang Biotechnology has turned a profit! March 2, 2026
- Jiuyuan Gene’s “Simeglucopyranoside” for weight loss (Jikeqin®) has been submitted for market approval March 2, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



