Opportunity moment for Chinese ADC
May 20, 2025
Source: drugdu
 258
258
Recently, Eli Lilly announced that its Nectin-4 targeted ADC drug LY4052031 has suspended clinical trials due to safety issues.
Nectin-4 is a hot target for ADC drug development. Currently, only one Nectin-4 targeted ADC product has been approved for marketing in the world, namely Padcev, which is approved for the treatment of urothelial carcinoma. Eli Lilly has deployed two next-generation anti-nectin-4 ADC drugs: LY4052031 (the toxin is the DNA topoisomerase I inhibitor camp98) and LY4101174 (the toxin is exatecan). This suspension involves LY4052031, while LY4101174 is still in Phase I clinical trials, and its efficacy and toxicity in humans remain to be observed.
This incident also aroused the industry's attention to the safety of Nectin-4 ADC, and exposed the difficulties in the research and development of ADC drugs: payload toxicity control, linker stability, any "shortcomings" in any link may trigger a chain reaction.
1. China's Biotech industry faces opportunities
Nectin-4 is an immunoglobulin-like antigen. Normal tissues only express low to moderate levels of Nectin-4, but it is overexpressed in a variety of cancers, including urothelial carcinoma, breast cancer, ovarian cancer, non-small cell lung cancer, and gastric cancer. At the same time, in retrospective studies, high expression of Nectin-4 is associated with poor prognosis, which also means that it can be used to improve patient prognosis. This makes the protein an attractive target for cancer treatment.
Currently, only one Nectin-4 ADC has been approved for marketing in the world, namely enfortumab vedotin (Padcev) from Astellas and Pfizer. The drug was first approved in the United States in 2019 for the second-line treatment of advanced urothelial carcinoma; in 2023, it was approved for combination with Keytruda for the first-line treatment of patients with locally advanced or metastatic urothelial carcinoma.
Padcev's sales exceeded $1 billion in 2023, and reached $1.588 billion in 2024, a year-on-year increase of more than 50%. According to Nature Reviews, Padcev's sales are expected to reach $3.5 billion by 2026.
The success of Padcev in urothelial carcinoma demonstrates the clinical application value of Nectin-4-targeted ADC.
However, Padcev comes from Seagen's second-generation antibody conjugation technology, which has limitations such as uneven conjugation ratios, which can lead to drug heterogeneity. Its instructions carry a black box warning that can cause severe and fatal skin adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). Such skin adverse reactions mainly occur in the first cycle of treatment, but may also occur later. In addition, Padcev has limited efficacy in cancers other than urothelial carcinoma, which limits the scope of its clinical indications.
Therefore, various companies have tried to optimize the toxins and linkers to develop a new generation of Nectin-4 ADC drugs and expand their clinical application range.
Among them, Mywell Biopharma's 9MW2821 has made the fastest progress and has entered Phase III clinical trials.
9MW2821 applies two global patents of drug-containing linker and site-specific coupling technology of its ADC development platform, uses cysteine bridge site-specific coupling to form ADC drugs with a uniform DAR value of 4, and uses the site-specific linker linker IDconnect to replace maleimide (MC) for better stability. Compared with Padcev, 9MW2821 has greatly improved the common skin toxicity, eye toxicity and peripheral neuropathy in Nectin-4 targeted therapy.
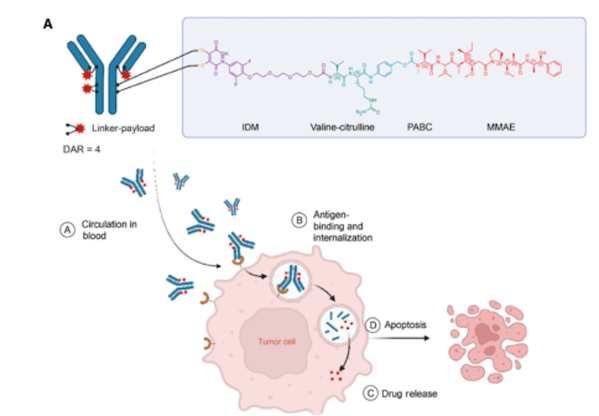
Image source: Mywell Biopharm
Currently, 9MW2821 has shown excellent efficacy and good safety in multiple indications including urothelial carcinoma (UC), triple-negative breast cancer (TNBC), esophageal cancer (EC), and cervical cancer (CC).
For urothelial carcinoma, which is the main target of Padcev, the objective response rate (ORR) and disease control rate (DCR) of 9MW2821 monotherapy as a second-line treatment were 62.2% and 91.9%, respectively, which are better than Padcev's 41% and 71.9%.
The combination of 9MW2821 and PD-1 monoclonal antibody for the first-line treatment of urothelial carcinoma patients has also entered the Phase III clinical stage. Data showed that the objective response rate (ORR) of this combination therapy was 87.5%, the confirmed ORR was 80%, and the disease control rate (DCR) was 92.5%, which were excellent data.
In terms of safety, data from nearly 260 patients treated with 9MW2821 published at ASCO 2024 showed that the safety was generally controllable. The most common adverse reactions were leukopenia and neutropenia (nearly 50% of patients), of which 25% of patients experienced grade 3-4 adverse reactions, which could be effectively controlled by G-CSF.
At present, 9MW2821 has entered clinical phase III for UC and CC indications, and phase II for EC and TNBC indications. It has been granted fast track designation by the FDA (cervical cancer, esophageal squamous cell carcinoma, triple-negative breast cancer), orphan drug designation (esophageal cancer), and has been included in the list of breakthrough therapeutic products by the NMPA CDE (urothelial carcinoma). Pacific Securities expects that the total peak sales of multiple indications in China in the future are expected to reach 3.5 billion yuan.
In addition, the ADC drugs currently developed in China for multiple tumor types (such as breast cancer, cervical cancer, and esophageal cancer) mostly use camptothecin toxins. With the widespread use of similar drugs, the problem of drug resistance may become prominent in the future. 9MW2821 uses MMAE as a payload, showing a differentiated competitive advantage in these indications, creating an opportunity for it to enter the field of TOPi treatment. For example, Maiwei Bio is focusing on promoting the Phase II clinical trial of TOPi treatment of TNBC indications.
2. Platform advantages are fully demonstrated
The success of 9MW2821 stems from the new generation ADC site-directed conjugation technology platform (IDDC™ platform) and the new payload Mtoxin™ developed by Maiwei Biopharma.
Mywell Biopharma showcased the platform and the development of multiple ADC drugs enabled by the platform at the 14th World Antibody Drug Conjugate Conference (World ADC London).
IDDC™ consists of a number of systematic core patented technologies:
1. DARfinity™, a site-specific conjugation process, produces site-specific conjugated drugs with DAR 4 as the main component (DAR 4 ≧ 95%).
2. IDconnect™, a site-specific coupling linker, is a new type of site-specific coupling linker. The self-hydrolyzed structure of the designed linker can effectively inhibit the thiol exchange during drug metabolism, thereby improving the plasma stability of ADC drugs and increasing the load transfer efficiency (40% higher than the control group).
3. Conditional release structure LysOnly™: It is a new type of linker structure that relies only on lysinase release, which can enhance the tumor-specific release ability of ADC drugs and reduce off-target effects.
4. Novel payload Mtoxin™ (MF6): It is a camptothecin derivative developed by Maiwei Biotechnology. Compared with the known camptothecin molecules Dxd and SN38, this molecule has stronger tumor inhibition and better bystander killing effect, and maintains significant efficacy in the DXd-resistant multidrug resistance model.
Maiwei Biopharma's innovations are distributed in all aspects of ADC, especially its new payload MF6, which has been applied in three pipeline products: 7MW3711, 9MW2921, and 7MW4911:
7MW3711 (B7-H3 ADC)
B7-H3 is a hot target in the field of ADC research and development in recent years. It is overexpressed in various tumor tissues such as non-small cell lung cancer, pancreatic cancer, and primary liver cancer. Previously, Hansoh Pharmaceuticals reached an exclusive licensing agreement with GSK for more than US$1.7 billion for HS-20093, which successfully "went overseas" and attracted the industry's attention to this target.
Currently, there is no B7-H3 ADC on the market in the world. Mywell Biopharma's B7-H3 ADC (7MW3711) is in Phase I/II clinical trials. The combination of 7MW3711 and PD-1 inhibitors was also approved by NMPA this year for Phase Ib/II clinical trials in patients with advanced solid tumors. The drug was granted orphan drug status by the U.S. FDA in July 2024 for the treatment of small cell lung cancer.
At this year's AACR Annual Meeting, Mywell Biopharma presented preclinical research results on the combination of 7MW3711 and PARP inhibitors. The Phase I/II clinical research data of 7MW3711 monotherapy for advanced solid tumors and lung cancer will be announced at this year's ASCO conference.
9MW2921 (TROP2 ADC)
TROP2 is a mature target for ADC drug development. Compared with similar ADC products under development at home and abroad, Maiwei Bio's 9MW2921 has been significantly improved and enhanced in terms of endocytic activity, plasma stability, drug release characteristics, and bystander killing effects. It is currently in Phase I/II clinical trial stage. This year's ASCO conference will also announce for the first time its first human clinical study data for patients with advanced solid tumors.
7MW4911 (CDH17 ADC)
CDH17 is also a popular anti-tumor research target. It is overexpressed and redistributed in 50%-90% of gastrointestinal cancers, causing it to be exposed on the surface of cancer cells. Therefore, it becomes easier to be targeted by antibody drugs and becomes a potential ideal ADC target for the treatment of gastrointestinal tumors.
However, most of the current CDH17 ADC drug research and development is still in the preclinical development or early clinical stage, and the fastest progressing ones are only in the clinical phase I ramp-up stage. Preclinical studies of Mywell Biopharma's CDH17 ADC (7MW4911) showed significant anti-tumor activity in CDX/PDX models of various gastrointestinal tumors. In the CRC PDX model, significant anti-tumor activity was observed at 1-3 mg/kg administration; in the gastric cancer CDX model, 3 mg/kg showed significant anti-tumor activity. It is not interfered by the (PgP) resistance mechanism and has significant effects on multidrug-resistant gastrointestinal tumor models. In addition, toxicology studies showed good safety. Maiwei Biopharmaceuticals plans to file dual IND applications in China and the United States in the second half of this year.
Although Maiwei Biopharma has made innovations in many areas of ADC technology, it intends to do more than that.
Recently, Mywell Biopharma has successively reached cooperation with Insilico Medicine and Shenzhen Shida Technology to deploy AI to empower various links such as early target discovery and evaluation, ADC new toxin development, and large molecule drugability optimization, comprehensively accelerating the innovative research and development process of ADC drugs.
3. Conclusion
In the ADC R&D wave, although there are many participants, there are still very few companies that can successfully develop safe and effective ADC drugs and bring them to market. Currently, only 18 ADC drugs have been approved for marketing worldwide. As a shining star in the era of precision medicine, the design concept of ADC seems intuitive - to accurately deliver highly effective cytotoxic drugs to tumor cells through specific antibodies to achieve "precision strikes", but in actual operation, every link - antibodies, linkers, toxins need to be carefully designed to truly realize its huge potential.
As an innovative biopharmaceutical company, R&D innovation is the core driving force and the future. With continuous technological innovation and clinical-oriented indication selection, Maiwei Biopharma is constantly building its unique advantages in the ADC track. In the future, with the empowerment of AI technology, Maiwei Biopharma is expected to take the development of new ADC targets, new toxins and new ADC technology platforms to a higher level and become an innovative leader in the ADC field.
https://news.yaozh.com/archive/45471.html
By editorRead more on
- Rovaxitinib approved for marketing, filling the demand for myelofibrosis treatment March 2, 2026
- Warrant Pharmaceuticals’ active pharmaceutical ingredient receives Brazil’s first official GMP certification March 2, 2026
- Merck’s New Story March 2, 2026
- Rongchang Biotechnology has turned a profit! March 2, 2026
- Jiuyuan Gene’s “Simeglucopyranoside” for weight loss (Jikeqin®) has been submitted for market approval March 2, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



