Small nucleic acid therapy challenges GLP-1 giants
July 10, 2025
Source: drugdu
 198
198
RNAi technology targets the core shortcomings of GLP-1 drugs by precisely regulating the expression of obesity-related genes. With the disruptive advantage of "reducing fat without losing muscle", RNAi therapy is strongly challenging the "hegemony" of the GLP-1 market worth hundreds of billions!
01The wave of small nucleic acid therapy has arrived
Small nucleic acid therapy, especially siRNA (small interfering RNA) technology, with its unique gene silencing mechanism and continuously innovative delivery technology, jointly promotes the expansion of treatment areas from rare diseases to chronic diseases, leading the third wave of innovation in the biomedicine field.
From the technical principle, siRNA achieves precise gene silencing through the RNA interference (RNAi) mechanism. In its double-stranded structure, the guide strand (antisense strand) is loaded into the RISC complex and then specifically binds to the target mRNA and guides its degradation, thereby blocking the expression of pathogenic proteins; while the guest strand (sense strand) plays a protective delivery function. What is particularly critical is that the RISC complex can be recycled, and a single siRNA molecule can continuously cut multiple mRNA targets, giving the drug long-lasting action characteristics (Figure 1). This mechanism breaks through the limitations of traditional drugs, making previously "undruggable" targets possible, and significantly broadening the range of indications.
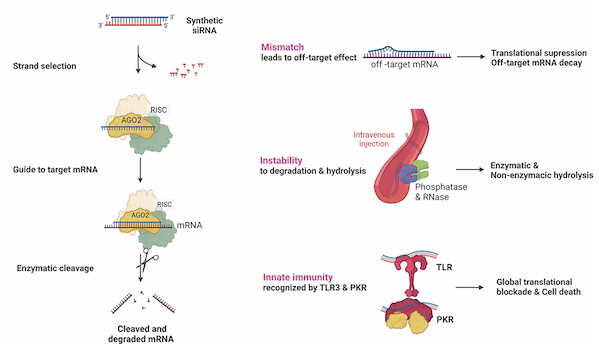 Figure 1 Mechanism and characteristics of siRNA
Figure 1 Mechanism and characteristics of siRNA
Image source: Reference [1]
The progress of industrial development has confirmed the conversion efficiency of siRNA technology breakthroughs. In 2018, the world's first siRNA drug Patisiran was approved, marking the official launch of RNAi therapy; in 2019, the GalNAc-coupled delivery technology drug Givosiran was launched, which systematically solved the stability and toxicity problems of early siRNA through its efficient liver targeting and sustained-release properties; in 2020, the cholesterol-lowering drug Inclisiran was approved by the European Union, extending its indications from rare diseases to the field of chronic cardiovascular diseases, opening up a new market space.
To date, seven siRNA drugs have been approved by the FDA for marketing worldwide. Except for the first drug that uses LNP as a delivery system, the other six all adopt the GalNAc delivery system, which fully verifies the clinical advantages of this technology platform. Its subcutaneous injection method is both long-lasting and safe, and its liver targeting efficiency exceeds 90%, providing a revolutionary treatment option for liver diseases.
In addition, market growth data further proves the explosive trend of the small nucleic acid drug industry. According to Evaluate Pharma statistics, the global siRNA drug market has exceeded US$5 billion and is expected to reach US$20 billion in 2030. This growth momentum is not only due to the success of existing liver-targeted drugs, but also benefits from the expansion of treatment areas brought about by technological iteration. With the development of new delivery systems, siRNA therapy is breaking through the limitations of the liver and expanding to multiple organ diseases such as the kidneys, central nervous system and tumors, continuously injecting development potential into the industry.
02Lock on the "golden target" of obesity
In recent years, based on large-scale human genetics research, scientists have successfully identified several genetically verified "golden targets" for obesity treatment, providing a new direction for the development of a new generation of weight loss therapies.
Pathogenic mutations in GPR75 are important genetic factors that reduce the risk of obesity. An exome sequencing study covering 645,000 people published in the journal Science in 2021 revealed (Figure 2): Individuals carrying GPR75 loss-of-function mutations (about 1/3000) lost an average of 5.3 kg and had a 54% lower risk of obesity. Mechanistic studies have shown that GPR75 is highly expressed in appetite-regulating neurons in the hypothalamus and regulates energy intake balance. Animal experiments further confirmed that mice with GPR75 gene knockout had a 44% lower weight gain rate on a high-fat diet, and significantly improved glucose tolerance and insulin sensitivity.
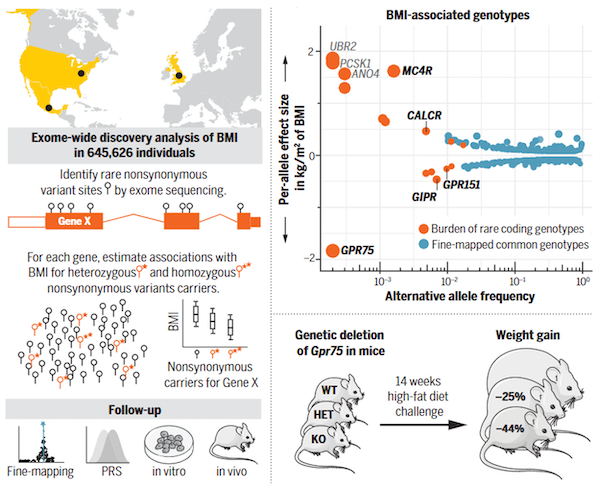 Figure 2 Discovery of BMI-related genes based on exome sequencing
Figure 2 Discovery of BMI-related genes based on exome sequencing
Coincidentally, a genetic study published in Nature Communications in 2022 found that carriers of INHBE loss-of-function mutations showed improved metabolic characteristics such as low waist-to-hip ratio (i.e. reduced abdominal fat), low triglycerides, and increased high-density lipoprotein.
INHBE and ALK7 belong to the TGFβ superfamily and constitute the metabolic regulation axis between the liver and adipose tissue. INHBE is expressed in the liver, and its product Activin E is secreted into the blood, binds to the ALK7 receptor on the surface of adipocytes, activates the Smad2/3 signaling pathway, and then inhibits lipolysis, promotes lipid storage, and induces insulin resistance (Figure 3).
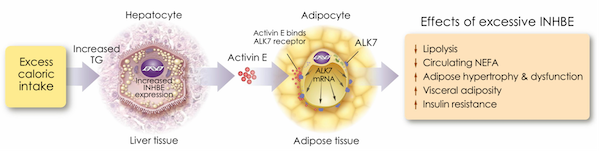 Figure 3 Hepatic activin E encoded by the INHBE gene regulates energy homeostasis in adipose tissue
Figure 3 Hepatic activin E encoded by the INHBE gene regulates energy homeostasis in adipose tissue
In addition, lipoprotein lipase (LPL) plays an important role in triglyceride (TG) metabolism and fat distribution. ANGPTL3/4/8 determines the storage and utilization of TG by finely regulating LPL activity. Among them, ANGPTL3 is continuously expressed in the liver, inhibiting LPL activity, increasing plasma VLDL levels, and promoting atherosclerosis.
Mechanistic studies have shown that ANGPTL4 is upregulated during fasting, inhibiting LPL in white adipose tissue (WAT), prompting TG to be used by the heart and muscles; ANGPTL8 is upregulated during eating, forming a complex with ANGPTL3 to inhibit muscle LPL and drive TG to be stored in WAT. Therefore, ANGPTLs have also become a potential target for a new generation of lipid-lowering drugs that domestic and foreign companies are competing to deploy.
03Touching the cake of GLP-1
Small nucleic acid drugs are entering the 100 billion weight loss market with a disruptive attitude, directly targeting the clinical defects and market gaps of current GLP-1 receptor agonists. In 2024, the global sales of semaglutide reached 29.296 billion US dollars, and the growth rate of telportide was nearly 220%, confirming the dominant position of GLP-1 drugs. However, its pain points are significant. The weight rebound rate after discontinuation of the drug exceeds 80%, and weight loss is accompanied by about 25%-40% muscle loss, resulting in a decrease in metabolic rate and the risk of weakness.
In response to these shortcomings, small nucleic acid drugs with RNAi as the core have broken through through a new mechanism. Data show that among the 20 small nucleic acid weight loss pipelines in the world, 6 have entered Phase I clinical stages or above, and another 14 are in the preclinical research stage (Figure 4).
Among many targets, INHBE is considered a potential "golden target" for fat loss. Silencing INHBE can activate the lipolysis pathway. Preclinical data showed that the weight loss effect of INHBE-SiRNA alone was comparable to that of semaglutide, while the muscle retention rate was increased by more than 90%. Arrowhead's ARO-INHBE achieved target knockdown for more than 85 days after a single administration in the crab-eating macaque model, and fat mass decreased significantly.
In addition, long-term efficacy is another significant advantage of small nucleic acid drugs. For example, Alnylam supports subcutaneous injection once every 3-6 months based on the GEMINI platform, while Wave's WVE-007 only requires 1-2 administrations, which breaks through the compliance bottleneck caused by GLP-1 requiring weekly injections.
What is particularly critical is that small nucleic acid drugs and GLP-1 show synergistic potential, and the combination of the two can form a dual pathway of "fat reduction and food suppression". Wave experiments have shown that its INHBE siRNA combined with semaglutide doubled the weight loss effect of mice and prevented 83% of weight rebound after GLP-1 was discontinued. Arrowhead has directly incorporated the combination of ALK7 siRNA and tirpotide into the design of Phase I/IIa clinical trials.
In the fierce competition of R&D, technology platform has become the key to success. Arrowhead uses TRiM delivery system to achieve adipose tissue targeting, while advancing the dual pipelines of ARO-INHBE and ARO-ALK7; Wave uses GalNAc coupling technology to enable WVE-007 to start the INLIGHT Phase I trial in 2025, and human data is expected to be released within the year; Alnylam uses GEMINI multi-target platform to deploy INHBE combination therapy.
04 Conclusion
Although small nucleic acid drugs are coming on strong, the competition threshold in the industry is rising rapidly as more and more players enter the market, and the difficulty of getting a piece of the pie is escalating. Small nucleic acid companies need to continue to make breakthroughs in delivery efficiency and cost control in order to share in the 100 billion market.
https://news.yaozh.com/archive/45734.html
By editorRead more on
- Rovaxitinib approved for marketing, filling the demand for myelofibrosis treatment March 2, 2026
- Warrant Pharmaceuticals’ active pharmaceutical ingredient receives Brazil’s first official GMP certification March 2, 2026
- Merck’s New Story March 2, 2026
- Rongchang Biotechnology has turned a profit! March 2, 2026
- Jiuyuan Gene’s “Simeglucopyranoside” for weight loss (Jikeqin®) has been submitted for market approval March 2, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



