Ddu College——WHO vaccine pre-certification How to conduct effective consultation?
August 25, 2021
Source: drugdu
 1,927
1,927

From November 1st to 2nd, 2018, the annual World Health Organization (WHO) Prequalification (PQ) consultation will be held in Kunming, China. Unlike previous years, this year's consultation will only take the one-on-one format, namely the World Health Organization Vaccine Pre-Certification Review Team and small meetings of various domestic vaccine manufacturers. Although the part of the centralized consultation meeting will be cancelled, which means companies and international organizations are unable to discuss and share the importance of pre-certification, but it’s believed that after several years of consultations, everyone has already cooked up the importance and significance of pre-certification. Evidence? This year's 14 vaccine manufacturers actively participate in the one-on-one consultation is the best proof.
As of November 2018, there were 4 manufacturers and vaccines that successfully passed the pre-certification of vaccines in China, namely, Chengdu institution’s Yinao, Hualan's Liugan, Kexing's Miejia and Beishengyan's bOPV.
At the PQ consultation meeting in Beijing last year, Ddu was fortunate to hear the pre-certification experience sharing between Kexing and Beishengyan. The biggest achievement was to realize the importance of the pre-submission meeting. That is, before the formal PQ declaration, the company needs to conduct multiple communication and consultation with the WHO review team to ensure understanding of the requirements of the WHO pre-certification, and to conduct gap analysis according to the actual situation of the enterprise, and then hire an expert consultant team to carry out the consult service.
Then what Ddu wants to say to everyone is how to do an effective consultation?
First of all, companies need to specify whether the vaccine to be declared for PQ is included in the Vaccines prequalification priority list. If it is included, it can be declared. If it is not included, companies can also communicate with the WHO review team. The WHO review team will take the medical product itself, the international needs and many other aspects into consideration, then make the decision about whether to accept the company's declaration.
Secondly, after determining the eligibility for declaring, companies need to fully understand the basic processes and requirements for WHO vaccine pre-certification (see Annexes I and II). In order to better promote the participation of Chinese vaccine manufacturers in the pre-certification of the WHO, the WHO Office in China has set up an expanded immunization planning group to support the pre-certification declaration projects of domestic vaccine manufacturers. Enterprises should actively contact the WHO Office in China to communicate with each other, accurately understand the pros and cons of vaccine pre-certification, processes, and requirements, and determine whether to initiate pre-certification projects after repeated consultations.
Thirdly, after confirming the start of the pre-certification project, the company should prepare a comprehensive consultation of the manufacturer and the product. Specifically, it includes preparing a set of product documents to be declared for PQ, covering all aspects of its plant facilities, research, and development, production, quality control, stability, non-clinical, clinical, etc., and preparing a PPT and consultation list for an introduction. Introduce the basic situation of the product through one-on-one consultation with the WHO review team. Preliminary assessment of the gaps and possibilities of PQ filings through questions and forms of questions and answers.
Next, based on the results of the comprehensive consultation, the company conducts a gap analysis of the existing situation, clarifies the focus and priority of the next project along with the conduction of specific consultation. Usually, the gaps among the enterprises are in the clinical aspect, such as the lack of continuous three batches of consistency in production scale, non-inferiority, combined vaccination, and other tests and data. Therefore, clinical consultation should be given priority to determine which clinical trials need to be supplemented. At the same time, companies should also hire a team of consultants to conduct special GMP simulation inspections and quality consultations to assess whether there are process changes or workshop modifications.
Finally, after both comprehensive consultation and specific consultation, the company can clearly recognize its own gaps and make necessary rectifications to meet the requirements of the WHO pre-certification declaration. Therefore, an effective pre-submission meeting is essential, and companies should fully grasp the opportunity of each pre-submission meeting!
Ddu hopes that everyone can learn more about WHO vaccine pre-certification after reading this article, so that more and more companies can successfully pass pre-certification, let our excellent vaccine varieties go abroad and be recognized by more countries around the world!
Annex II
WHO Vaccine Pre-certification Description
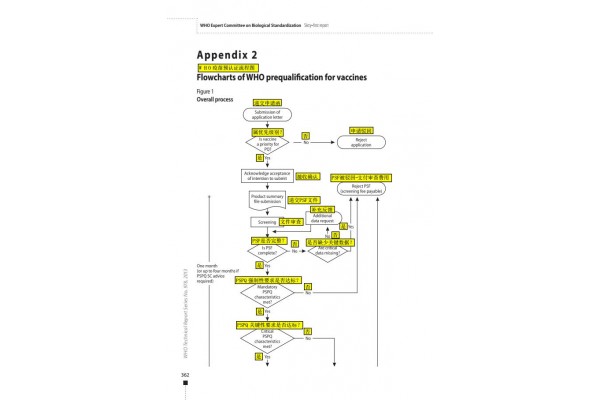
The process of WHO vaccine pre-certification is roughly divided into the following six steps:
1.Submit an application
Submit an application letter to express the intention of the manufacturer to conduct product pre-certification qualification assessment.
The time for submitting the application letter is not limited, but it is required to state the estimated time when the product documentation can be provided.
2.Seminar
The manufacturer, the head of the State Food and Drug Administration (if any), and the head of the WHO will participate in the discussion. At the meeting, the manufacturer can consult on the issues related to pre-certification.
Manufacturers can raise their own needs, and WHO arranges assessment meetings based on the needs of manufacturers.
3.Product summary file(PSF)
If the application letter is approved, the manufacturer must prepare and submit a product profile (1 paper file and 5 electronic files in CD format). The document is subject to the WHO format as follows:
■ Chapter 1: General information;
■ Chapter 2: Personnel;
■ Chapter 3: Premises and equipment;
■ Chapter 4: Vaccine composition, presentations and schedules;
■ Chapter 5: Production;
■ Chapter 6: Quality control;
■ Chapter 7: Stability;
■ Chapter 8: Clinical experience;
■ Chapter 9: Production and distribution data;
■ Chapter 10: Update on regulatory actions.
Remarks:
- Recognize and accept the CTD format. If the CTD format is used, the file should cover all the contents of the PSF.
- The time node for submitting product information is January 31, May 31 and September 31 of each year.
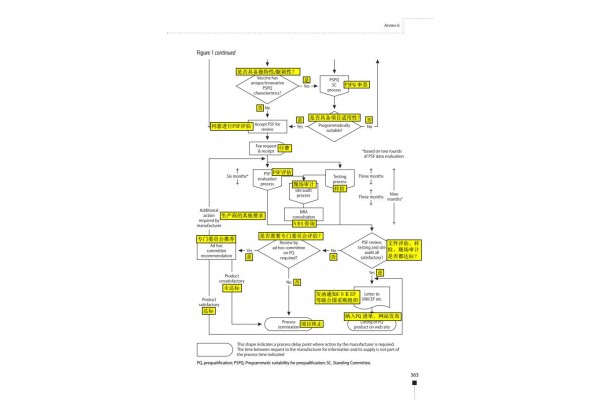
3.1 PSF document review (1-4 months)
(1) Review the completeness and standardization of PSF documents. There will be 2 rounds of document review.
If the first round does not pass, WHO will issue a formal notice to inform the manufacturer that the documents have been rejected and require the manufacturer to pay the corresponding review fee
If the first round of review is passed, there is no need to pay the review fee. After the second round of evaluation, the WHO will issue a formal notice to the manufacturer to review the document and ask the manufacturer to pay the corresponding review fee.
(2) Review the applicability of the project.
The Pre-certification Program Applicability Evaluation Committee (PSPQ) will evaluate the key attributes, uniqueness and innovation of the product. The recommendation letter of PSPQ is of great significance to the approval of WHO.
If the information is insufficient, the manufacturer must provide supplementary feedback to the expert's evaluation within one month for follow-up review. If passed, PSPQ will issue a formal notice to inform the manufacturer to pay the corresponding review fee.
If the review fails, PSPQ will issue a formal notice to inform the manufacturer that the application has been rejected and ask it to pay the corresponding review fee, and WHO will destroy the PSF file.
3.2 Evaluation of PSF documents (6 months)
(1) The first round of document evaluation takes 3 months. If the information is insufficient during the evaluation process, WHO will require the manufacturer to provide supplementary feedback (submitted in 1 paper file and 5 CD format electronic files). If the manufacturer fails to replenish within 3 months, WHO will terminate the assessment.
(2) The second round of document evaluation took 3 months to refer to the evaluation of the complete document after receiving additional feedback.
4 sample inspection (3 months from the date of submission of the sample)
After the PSF document review and evaluation is passed, the manufacturer must provide a certain number of samples (ranging from 25 to 200), requiring 3-5 batches in succession, as well as sample accompanying materials (batch record summary, test method SOP, reagent) , standard products, etc.)
5 On-site audit (taken within 2 months after passing the sample test)
The inspection report will be sent to the manufacturer within one month after the inspection, and will be copied to the State Food and Drug Administration. If corrections are needed, WHO will provide feedback on the rectifications, and manufacturers will need to submit feedback by the deadline.
6 results evaluation
If the assessment is approved, WHO will issue a letter to the UN agencies and relevant organizations stating that the vaccine product meets the requirements of the WHO and UN agencies, and is approved and confirmed by the SFDA, and a report will be presented to supplement the facts.
The vaccine will be included in the vaccine pre-certification list.
The pre-certification qualification is valid until the WHO initiates a reassessment.
By editorRead more on
- Anglikang Adenosylcobalamin Capsules Obtain Drug Registration Certificate March 6, 2026
- Two Minoxidil Topical Solution Applications from its Subsidiary Approved March 6, 2026
- Zhejiang Medicine’s ARX305 Initiates Phase II Clinical Trial March 6, 2026
- Indacaterol Mometasone Inhalation Powder Clinical Trial Approved for Asthma Treatment March 6, 2026
- Harsco Pharmaceutical’s innovative drug HSK50042 tablets receive clinical approval for a new indication March 6, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



