Should the clinical use of etomidate and research on its derivatives be stopped?
July 29, 2025
Source: drugdu
 248
248
Recently, a commentary article in the British Journal of Anaesthesia, “Etomidate and its derivatives: time to say goodbye?” [1], has attracted the interest of top experts in the field of anesthesia. The debate over the survival of etomidate, the “pioneer of anesthesia,” has unfolded in the journal.
Behind the advocacy of giving up
who played a key role in the clinical research of remimazolam [2,3,4] and was formerly the Director of the Council of the European Society of Anaesthesiology and Vice-Chairman of the Council of the Society of Anaesthesiologists of Great Britain and Ireland, and his collaborators argued in the article “Etomidate and its derivatives: time to say goodbye?” that etomidate is not competitive with propofol and remimazolam and that the use of etomidate and research on its derivatives should be stopped.
He claimed that etomidate not only inhibits adrenal cortex hormones, but also easily induces myoclonus. In the more than 20 years of research and development of etomidate derivatives, except for Carbo-etomidate, which has almost no efficacy, and MOC-etomidate, which has a prolonged awakening time and does not inhibit corticosteroids (author's note: in fact, the latter should have a shorter inhibition time [5]), other derivatives such as CPMM and ET-26 not only fail to completely eliminate the side effect of inhibiting adrenal cortex hormones, but also induce more severe myoclonus.
The clash of different viewpoints
Dr. Evan D. Kharasch, former vice president and director of the Clinical Translational Research Laboratory at the University of Washington, editor-in-chief of the journal Anesthesiology, and distinguished professor at Duke University, and Dr. Per-Arne Lönnqvist, professor of pharmacology at the Karolinska Institute in Sweden and senior pediatric anesthesiologist, hold different views. They point out that etomidate's stable hemodynamic properties are significantly better than other anesthesia induction drugs, and its role in anesthesia induction is irreplaceable for patients with hemodynamic instability or cardiovascular disease, especially frail and elderly patients.
They believe that the adrenocortical suppression caused by induction doses of etomidate is transient and reversible. Each drug has its desirable advantages and unpleasant side effects. The hemodynamic stabilization effect of etomidate during anesthesia induction is unmatched by other anesthetic drugs such as propofol and remimazolam. They believe that there is no clear evidence that induction doses of etomidate will cause irreversible adrenocortical suppression, and there is no evidence that myoclonus occurring during induction is uncontrollable [6,7] .
The author's thoughts and insights
Professor Sneyd's "farewell declaration" to etomidate is not groundless. Etomidate does have defects such as suppressing adrenal cortex hormones and myoclonus, but the view that propofol and remimazolam can completely replace etomidate may be biased. Because there is no "perfect drug" among the current intravenous anesthetics, etomidate certainly has serious defects, and propofol, remimazolam and other intravenous anesthetics also have obvious shortcomings, all of which may cause serious consequences.
Propofol is widely used in the field of anesthesia due to its stable anesthetic state and rapid recovery. However, its impact on the circulatory system cannot be ignored [8,9] ; more importantly, long-term continuous infusion may cause fatal propofol infusion syndrome (PRIS) [10,11,12] . FDA data [13] show that in the United States alone, there were 47 cases of propofol infusion syndrome in 2024, of which 18 died, with a mortality rate of more than 38% (Figure 1). The European EMA EudraVigilance database shows that from June 6, 2024 to October 20, 2024, there were 13 new cases of PRIS in the European Economic Area (EEA), of which 6 resulted in death [ 14] , with a mortality rate of up to 46%!
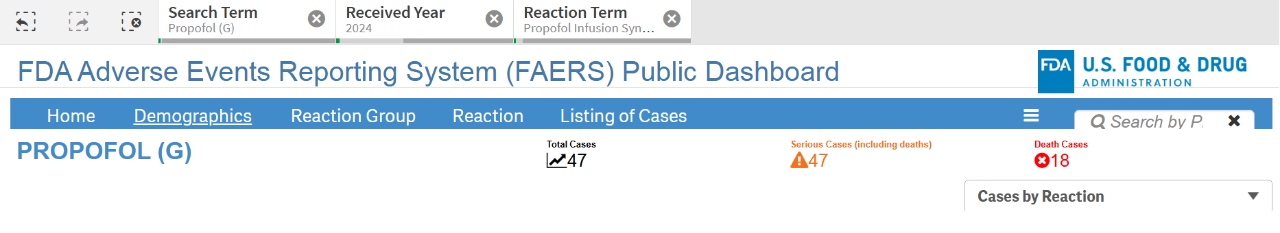
Figure 1 Propofol adverse event report data disclosed by FDA Image source: FDA
The new anesthetic drug remimazolam was approved for marketing in many countries around the world in 2020, but sales in different countries vary greatly. According to statistics [15] , in 2023, the sales of remimazolam in China were US$40.96 million, while in the United States they were only US$800,000. In Europe, where remimazolam originated, its sales were only about US$320,000. The sales in the two major markets of Europe and the United States, which have always been the most welcoming to new drugs, are less than 3% of the sales in the Chinese market!
The sales performance of remimazolam in the Chinese and Western markets is very different, and there may be some concerns behind it. From the molecular structure, it can be predicted that after intravenous injection of remimazolam into the body, it will be hydrolyzed by CES1 in the liver into carboxylic acid and methanol [16,17] . Methanol will be further oxidized into formaldehyde by dehydrogenase in the liver [18] (Figure 2); formaldehyde is active and easily condenses with amino groups on DNA, thus posing a risk of cancer [19] . This may also be the reason why the FDA restricts remimazolam to infusion within 0.5 hours [20] .
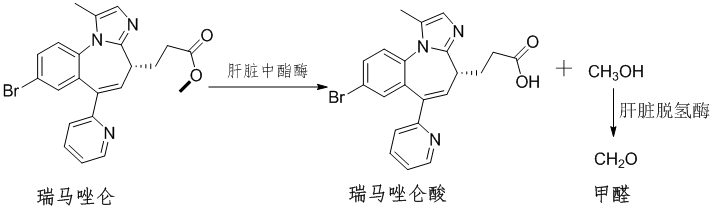
Figure 2 Metabolic process of remimazolam
Using etomidate for induction anesthesia can not only maintain the patient's hemodynamic stability, but also will not endanger the patient's life due to short-term corticosteroid suppression. Etomidate has not been withdrawn from clinical practice after half a century, which shows that it is irreplaceable for patients with cardiovascular and cerebrovascular diseases.
Professors Kharasch and Lönnqvist are undoubtedly correct in saying that etomidate should not be withdrawn from the market until better anesthetics that can maintain hemodynamic stability are available. Giving up lightly could cause the anesthesia field to fall into an "orphan drug" crisis, with no drugs available at critical moments.
The existing defects of etomidate and the progress of CPMM and ET-26 have pointed out the direction for anesthesiologists and researchers to work on. In order to provide patients with safer anesthesia solutions, how to further enhance the strengths and avoid the weaknesses, and the exploration of derivatives that retain the unique hemodynamic advantages of etomidate while not inhibiting corticosteroids and inducing myoclonus cannot be stopped.
There are always more solutions than problems, and we believe that safer and more ideal anesthetic drugs will eventually appear.
Source: https://news.yaozh.com/archive/45837.html
By editor
Read more on
- Rovaxitinib approved for marketing, filling the demand for myelofibrosis treatment March 2, 2026
- Warrant Pharmaceuticals’ active pharmaceutical ingredient receives Brazil’s first official GMP certification March 2, 2026
- Merck’s New Story March 2, 2026
- Rongchang Biotechnology has turned a profit! March 2, 2026
- Jiuyuan Gene’s “Simeglucopyranoside” for weight loss (Jikeqin®) has been submitted for market approval March 2, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



