Can AOC become the “next stop” for oligonucleotide therapy?
December 17, 2025
Source: drugdu
 92
92
In recent years, the nucleic acid industry has developed rapidly, with many small nucleic acid drugs successfully approved for marketing, bringing revolutionary breakthroughs in the treatment of rare diseases, infectious diseases and other fields, and propelling gene therapy into a golden age of rapid development. However, traditional small nucleic acid drugs still face many bottlenecks in the delivery process. For example, therapies represented by GalNAc delivery technology are mostly limited to liver targeting, making it difficult to achieve effective delivery to extrahepatic tissues such as muscles and the central nervous system. They also have problems such as short half-life and high off-target toxicity, which greatly limit their clinical application.
A few years ago, the emergence of AOC (antibody-oligonucleotide conjugate) initially solved the delivery problem that had previously hindered the development of small nucleic acid drugs. Its unique structural design and technological advantages have also made it the core direction of the "next stop" in the field of oligonucleotide therapy.
Now, after gaining a deeper understanding of the AOC structure, driven by strong capital from MNCs, and iterative upgrades of targeted delivery and coupling technologies, what will the present and future of AOC look like?
Each performs its own duties
The three major structures of AOC drugs
An AOC is a complex formed by linking an antibody to an oligonucleotide via a linker. It combines the targeting properties of an antibody with the therapeutic activity of an oligonucleotide. Structurally, it is similar to an ADC, except that the payload is replaced by a nucleic acid drug with gene regulation function instead of a small molecule toxin.
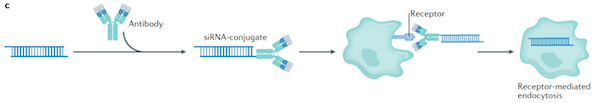 Image credit: Kalina Paunovska, et al. Drug delivery systems for RNA therapeutics
Image credit: Kalina Paunovska, et al. Drug delivery systems for RNA therapeutics
Compared to traditional oligonucleotide drugs delivered by GalNAc, AOC has three core advantages:
Firstly, there is the ability to deliver antibodies outside the liver. By selecting different targeted antibodies, precise delivery to extrahepatic tissues such as muscles, central nervous system, and tumors can be achieved. For example, AOC targeting TfR1 can penetrate the blood-brain barrier, providing new treatment options for central nervous system diseases such as Alzheimer's disease and spinal muscular atrophy.
Secondly, it has a longer half-life. Antibody molecules are more stable in the blood. The half-life of conjugated AOC can be several days to several weeks, which is much longer than that of traditional oligonucleotide drugs (usually several hours to several days), greatly reducing the frequency of administration.
Third, it has lower off-target toxicity. Since AOC only works on target cells that express specific antigens, it has minimal impact on normal cells, effectively reducing off-target effects such as immune responses and liver and kidney damage that may be caused by traditional oligonucleotide drugs.
In terms of mechanism, AOC has three clearly defined structural functions: antibodies are responsible for recognizing and mediating the specific internalization of target cells, ensuring that the drug is precisely located to the target tissue; linkers maintain in vivo stability and trigger the release of the payload in the cell, balancing the drug circulation stability and intracellular efficacy; while oligonucleotides achieve "gene silencing" or "splicing regulation" by specifically and complementaryly binding to target mRNA, thereby intervening in the disease process from the root.
As a next-generation precision therapy platform following ADCs, AOCs are rapidly developing due to their unique advantages in regulating gene expression levels. Several candidate drugs have already entered pivotal clinical stages, primarily for the treatment of hereditary and muscle-related diseases. With continuous technological iteration and the accumulation of clinical data, the future development of AOCs will become more diversified, potentially achieving breakthroughs in more therapeutic areas.
Multi-unit design: Achieving functional synergy and enhanced therapeutic effects
With the gradual maturation of "bispecific antibody" technology, the field has witnessed a milestone in drug feasibility validation. For example, Baili Tianheng's independently developed EGFR×HER3 bispecific antibody ADC drug, iza-bren, has submitted a marketing application in China for nasopharyngeal carcinoma. Correspondingly, future AOCs will no longer be limited to a single gene silencing function, but are expected to integrate multiple functional modules.
•
On the one hand, bispecific antibodies can be used as carriers to simultaneously target two different antigens or epitopes, such as targeting tumor cells at one end and immune cells at the other, to construct more complex AOCs and achieve stronger immune recruitment effects.
•
•
On the other hand, the design concept of dual-load ADCs can be adopted to simultaneously carry immune-activating oligonucleotides and gene-regulating oligonucleotides on the basis of AOC, so as to achieve the dual role of "treatment + immune regulation" and further improve the therapeutic effect, especially suitable for complex diseases such as tumors.
•
Expansion into the Oncology/Immunology Field: Opening Up a Broader Market Space
Generally speaking, the disease targets of AOC therapy are usually closely related to its target: Avidity's AOC-1001 and AOC-1004 products, because their antibody target is TfR1, are mostly indicated for diseases related to muscles, nerves, and other tissues, such as DMD and myotonic dystrophy; while Tallac's TAC-001 and ALX's ALTA-002 target CD22 and SIRPa, and their indications focus on solid tumors.
Clearly, target selection is the core factor limiting the scope of indications for AOC. Following muscle, central nervous system and tumor indications, autoimmune diseases may become the next important target for AOC therapy due to their huge market demand.
Currently, some companies in the market have begun to develop immune-activating active ingredients (AOCs). By conjugating immune-activating molecules such as TLR agonists and STING agonists, AOCs can activate the body's immune system while achieving targeted gene silencing, making them particularly suitable for cancer treatment. Furthermore, AOCs can be used in combination with immune checkpoint inhibitors to enhance the therapeutic effect of these inhibitors by silencing immunosuppression-related genes, providing new combination therapies for cancer treatment.
https://news.yaozh.com/archive/46635.html
By editorRead more on
- a full look at 15 blockbuster drugs. February 28, 2026
- EMA CHMP Recommends EU Approval of Henlius’ Pertuzumab Biosimilar HLX11 February 28, 2026
- Akeso Biopharma’s autoimmune pipeline reaches another milestone! Mandocizumab submits application for market approval, targeting a market worth tens of billions. February 28, 2026
- $2.1 billion! Novo Nordisk reaches new cooperation agreement February 28, 2026
- After Eli Lilly and Novo Nordisk, who most resembles the “Third Brother of Weight Loss”? February 28, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



