-
Chiatai Tianqing Achieves Positive Results in Pivotal Registration Study of Class 1 Innovator Rovadicitinib Tablets
- Source: drugdu
- 313
- April 22, 2024
-
Urine-Based Test Detects Head and Neck Cancer
- Source: drugdu
- 436
- April 20, 2024
-
Unique Hand-Warming Technology Supports High-Quality Fingertip Blood Sample Collection
- Source: drugdu
- 386
- April 20, 2024
-
Cybersecurity Firm Says it Has Removed Hundreds of Sites Selling Fake Weight Loss Drugs
- Source: drugdu
- 524
- April 20, 2024
-
GSK’s Five-in-One Meningococcal Vaccine Under Review by the FDA
- Source: drugdu
- 356
- April 20, 2024
-
Glenmark recalls 6,528 bottles of BP drug in US
- Source: drugdu
- 355
- April 20, 2024
-
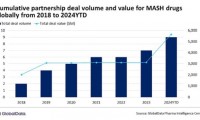
GlobalData
- Source: drugdu
- 556
- April 20, 2024
-
Chemomab touts study confirming CCL24 link to systemic sclerosis severity
- Source: drugdu
- 327
- April 20, 2024
-
Image-Based AI Shows Promise for Parasite Detection in Digitized Stool Samples
- Source: drugdu
- 397
- April 19, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.