-
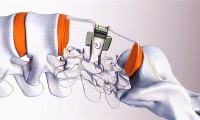
Orthofix has positive spine stimulation tech data
- Source: drugdu
- 182
- December 27, 2023
-
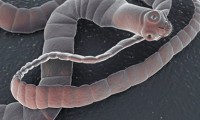
That Tingling in Your Leg could be a Worm in Your Spine
- Source: NBC News
- 711
- July 16, 2018
-
Centinel Spine acquires Prodisc assets from J&J’s DePuy Synthes
- Source: Massdevice
- 666
- September 20, 2017
-
7 Innovative Medical Devices Enter Special Review Process
- Source: drugdu
- 72
- October 23, 2024
-
Jianjia Robot applies to withdraw its IPO application documents
- Source: drugdu
- 100
- September 30, 2024
-
Medtronic and Siemens Healthineers reach a major collaboration
- Source: drugdu
- 70
- September 28, 2024
-
Former President of Johnson & Johnson Orthopedics jumps to orthopedic giant!
- Source: drugdu
- 76
- September 21, 2024
-
Orthopedic giant, head coach of Greater China region resigns
- Source: drugdu
- 52
- September 12, 2024
-
Donated 160 million yuan to the Qinghai-Tibet Plateau
- Source: https://mp.weixin.qq.com/
- 82
- September 11, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.