Major approval! The world’s first cAMP-biased GLP-1 inhibitor, Synvita’s “Enoglutide,” has been approved for marketing
February 3, 2026
Source: drugdu
 29
29
 Enoglutide Injection (formerly known as Enoglutide, code name XW003), independently developed by Hangzhou Xianweida Biotechnology , for the purpose of glycemic control in adults with type 2 diabetes.
Enoglutide Injection (formerly known as Enoglutide, code name XW003), independently developed by Hangzhou Xianweida Biotechnology , for the purpose of glycemic control in adults with type 2 diabetes.
This is Syntec's first commercialized product and the world's first approved cAMP-biased GLP-1 receptor agonist.
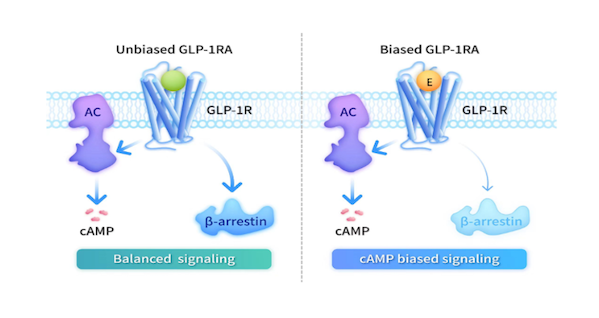
of enoglutide is its world-first cAMP-biased mechanism of action, which is the key difference between it and existing GLP-1 products.
Traditional GLP-1 products are "unbiased," and when they activate the beneficial cAMP signaling pathway, they recruit β-arrestin, leading to receptor desensitization and affecting long-term efficacy. In contrast, enoglutide, through precise molecular design, can selectively activate the cAMP pathway and minimize β-arrestin recruitment, maintaining continuous therapeutic signals. Its development is based on the biased activating theory/concept derived from the Nobel Prize-winning GPCR research , which has significant scientific implications.
 This product is a long-acting weekly formulation, administered via subcutaneous injection, and its activity and production capacity are enhanced by leveraging the proprietary technology platform of Xianweida. In addition to the type 2 diabetes indication approved this time, its marketing application for the adult overweight/obesity indication has been submitted (December 2024) , which is expected to achieve "dual use of one drug".
This product is a long-acting weekly formulation, administered via subcutaneous injection, and its activity and production capacity are enhanced by leveraging the proprietary technology platform of Xianweida. In addition to the type 2 diabetes indication approved this time, its marketing application for the adult overweight/obesity indication has been submitted (December 2024) , which is expected to achieve "dual use of one drug".
In addition to expanding its indications, First-in-Depth is also developing an oral version of enologin (XW004), which has already started Phase II clinical trials. In the future, it will build a product portfolio of "injection + oral".
The approval of enoglutide injection is based on two pivotal Phase III clinical trials (EECOH-1 and EECOH-2) in Chinese adults with type 2 diabetes. The results consistently demonstrated that , whether as monotherapy or in combination with metformin, enoglutide significantly and sustainably reduces HbA1c, while also reducing weight and improving metabolic parameters. The efficacy was maintained stably for up to 52 weeks, with good safety and tolerability. Specifically, EECOH-1 demonstrated that monotherapy with enoglutide was significantly superior to placebo in terms of glycemic control and target achievement rate, exhibiting a dose-dependent effect. EECOH-2 showed that combination therapy with enoglutide was superior to dulaglutide 1.5 mg overall in terms of glycemic control magnitude and target achievement rate.
The GLP-1 market is currently highly competitive but also rife with homogenization . Can enoglutide leverage its differentiated advantages to become a domestic benchmark and compete with international giants? We shall wait and see.
https://bydrug.pharmcube.com/news/detail/56ee18b1a98009f03815342d804cf312
By editorRead more on
- Its groundbreaking innovative drug, Ruxolitinib Phosphate Cream, has been approved for marketing in China. It is the first and only targeted drug approved in China for the treatment of vitiligo. February 3, 2026
- Lunan Pharmaceutical’s Bilastin Receives Marketing Approval in Japan February 3, 2026
- Sinovac Biotech expects its net profit to increase by 328.83% to 455.89% in 2025 February 3, 2026
- Evidence-based breakthrough! Traditional Chinese medicine for anti-fibrosis selected again for the National Science and Technology Major Project February 3, 2026
- Amgen terminates development of a Phase 3 clinical antibody drug February 3, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



