Xingmeng Biotech obtains its first international GMP certification, accelerating the global rollout of its innovative monoclonal antibody drug, Cribi®.
January 4, 2026
Source: drugdu
 91
91
 On December 31, 2025, Xingmeng Biopharmaceutical (Suzhou) Co., Ltd. ("Xingmeng Biopharmaceutical") announced that it had officially obtained the Good Manufacturing Practice (GMP) certificate for pharmaceuticals issued by the Turkish Pharmaceutical and Medical Devices Authority (TITCK) . This certification is not only Xingmeng Biopharmaceutical's first overseas GMP certificate , but also signifies that the production quality management system of its independently developed core product, Zamerovic-Mazoprevir Injection (trade name: Cribi®), has reached international standards, obtaining key qualifications for this innovative product to enter the mainstream global market.
On December 31, 2025, Xingmeng Biopharmaceutical (Suzhou) Co., Ltd. ("Xingmeng Biopharmaceutical") announced that it had officially obtained the Good Manufacturing Practice (GMP) certificate for pharmaceuticals issued by the Turkish Pharmaceutical and Medical Devices Authority (TITCK) . This certification is not only Xingmeng Biopharmaceutical's first overseas GMP certificate , but also signifies that the production quality management system of its independently developed core product, Zamerovic-Mazoprevir Injection (trade name: Cribi®), has reached international standards, obtaining key qualifications for this innovative product to enter the mainstream global market.
The official GMP compliance on-site inspection by the Turkish authorities was conducted from September 15th to 19th, 2025. Following a rigorous audit, TITCK announced at the closing meeting that the Xingmeng Suzhou production base had achieved the highest level of compliance, "Class I." According to procedure, TITCK officially issued the GMP certificate on December 18th, 2025.
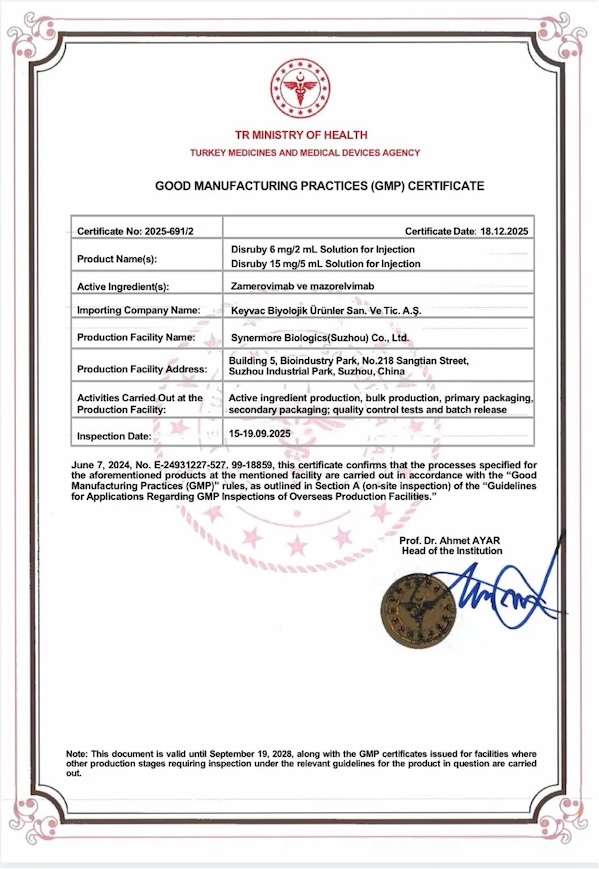 Image: GMP certificate issued by the Turkish Medicines and Medical Devices Authority (TITCK)
Image: GMP certificate issued by the Turkish Medicines and Medical Devices Authority (TITCK)
Zamelovir/Mazzorivir monoclonal antibody injection is a Class I innovative drug independently developed by Xingmeng Biotechnology in China. It is used for passive immunization in adults exposed to rabies virus and is the first anti-rabies cocktail monoclonal antibody in China that meets the recommendations of the World Health Organization (WHO). It was approved for marketing in China by the NMPA in June 2024. Krebi® is a mixture of equal parts zamelovir and macorivir. The two monoclonal antibodies bind discontinuous and non-overlapping epitopes, offering unique complementary advantages with different antigenic sites, effectively broadening the spectrum of virus neutralization. During its development, Krebi® strictly adhered to international standards, conducting clinical trials in multiple countries including China, the United States, and the Philippines to comprehensively evaluate the drug's safety and efficacy, and simultaneously applying for marketing approval in multiple countries/regions. Previously, Krebi® was granted "orphan drug" designation by the U.S. Food and Drug Administration, fully demonstrating its potential value and clinical application prospects globally.
As a member of the Pharmaceutical Inspection Cooperation (PIC/S) organization, Turkey's GMP standards are closely aligned with mainstream international regulatory systems. Xingmeng Bio's successful passing of the Turkish official inspection signifies that its production quality management system meets internationally recognized PIC/S standards, marking a crucial step in the internationalization of its core product, Krebi®. Building on this success, Xingmeng Bio will accelerate the global market registration process for Krebi®.
https://bydrug.pharmcube.com/news/detail/b617687a2340fd3af7f76aefb38ebd5e
By editorRead more on
- a full look at 15 blockbuster drugs. February 28, 2026
- EMA CHMP Recommends EU Approval of Henlius’ Pertuzumab Biosimilar HLX11 February 28, 2026
- Akeso Biopharma’s autoimmune pipeline reaches another milestone! Mandocizumab submits application for market approval, targeting a market worth tens of billions. February 28, 2026
- $2.1 billion! Novo Nordisk reaches new cooperation agreement February 28, 2026
- After Eli Lilly and Novo Nordisk, who most resembles the “Third Brother of Weight Loss”? February 28, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



