the legendary story of ketamine
September 23, 2025
Source: drugdu
 196
196
1. Ketamine, once known as a "party drug," is now reshaping the mental health field as a "miracle antidepressant." Its core advantage lies in its rapid onset, relieving symptoms of severe depression within hours. This drug is particularly revolutionary for patients with treatment-resistant depression (TRD) who are resistant to traditional medications and those experiencing acute suicidal ideation.
2. Johnson & Johnson's Spravato (esketamine nasal spray), a market pioneer, is expected to surpass $1.1 billion in sales in 2024, making it a blockbuster drug. However, its commercialization still faces three major challenges: strict regulation, high costs, and the risk of abuse.
3. In China, companies like Enhua Pharmaceutical and Hengrui Medicine are fiercely competing for the potentially safer and more effective R-ketamine. Beyond nasal sprays, companies around the world are also innovating in dosage forms like extended-release tablets. In clinical research, a team led by Dr. Wang Gang, President of Beijing Anding Hospital, has made significant contributions, promoting the standardized use of this therapy in China. While ketamine's efficacy is significant, its mechanism of action remains under investigation, and long-term efficacy and side effects remain key research areas.
1. Ketamine’s Past and Present: An Unexpected Journey from Anesthetic to Antidepressant
The legend of ketamine began in the 1960s, and its very creation was rife with drama. It wasn't invented out of thin air; rather, it was developed as a replacement for another powerful anesthetic, phencyclidine (PCP). While PCP offers powerful anesthetic effects, its side effects are equally daunting, often causing patients to experience hyperexcitement, delirium, and even schizophrenia-like symptoms, which significantly limits its clinical application. Scientists urgently needed an improved version of the drug that would retain PCP's anesthetic and analgesic effects while significantly reducing its psychiatric side effects. Against this backdrop, ketamine was successfully synthesized in 1962, perfectly inheriting the advantages of PCP while avoiding its most dangerous flaws.
Ketamine is unique in its ability to induce a state known as dissociative anesthesia. While patients appear awake and even maintain some reflexes, their consciousness is disconnected from their bodily sensations and the external environment, rendering them insensitive to pain. This property makes it a relatively safe anesthetic, particularly in resource-scarce or harsh environments. In 1970, the U.S. Food and Drug Administration (FDA) officially approved ketamine for clinical use, and it quickly became one of the most commonly used anesthetics in the United States. One of its most prominent applications was on the battlefields of the Vietnam War. Faced with a large number of casualties requiring emergency surgery, ketamine, with its rapid onset, minimal respiratory and circulatory depressant effects, and high safety profile, became a lifesaver for battlefield doctors, saving countless lives. In 1985, the World Health Organization (WHO) added it to its "Essential Medicines List," and it continues to play an indispensable role in global healthcare, particularly in pediatric and veterinary anesthesia.
If ketamine's journey as an anesthetic marked its first half, then its discovery in the field of antidepressant medicine marked its controversial second half. The origins of this shift can be traced back to a groundbreaking study at Yale University in 2000. While exploring the relationship between the glutamatergic nervous system and depression, a research team led by Professor John Krystal serendipitously discovered that low-dose ketamine could produce a rapid and potent antidepressant effect. This discovery completely overturned the traditional approach to antidepressant drug development. Since the 1950s, antidepressant drug development has revolved around the "monoamine hypothesis," which posits that depression is caused by insufficient levels of monoamine neurotransmitters (such as serotonin and norepinephrine) in the brain. Consequently, nearly all traditional antidepressants, such as selective serotonin reuptake inhibitors (SSRIs), aim to increase monoamine levels in the synaptic cleft. However, these drugs often suffer from slow onset of action (often taking weeks or even months) and poor efficacy in patients with treatment-resistant depression (TRD). Ketamine acts not on the monoamine system but, as an antagonist of N-methyl-D-aspartate (NMDA) receptors, directly on the glutamatergic nervous system. Crucially, its antidepressant effects are almost immediate. Studies have shown that after a single intravenous injection of ketamine, the average onset of action is just 1.5 hours, with peak effects occurring within 3 hours. Many patients experience a significant improvement in mood within hours, which is undoubtedly a revolutionary breakthrough for those suffering from severe depression and suicidal thoughts.
2. The "blockbuster" in the 10 billion market and the rise of domestic competitors
Ketamine's unique pharmacological mechanism and remarkable clinical efficacy have not only brought hope to countless patients struggling with traditional antidepressant treatments, but have also spawned a new market with enormous potential. Depression affects a vast number of people worldwide. According to the World Health Organization (WHO), approximately 350 million people suffer from it worldwide, and in China, this figure reaches 95 million. Traditional antidepressants are slow to take effect, often taking weeks or even months to show results, and are ineffective for approximately one-third of patients with treatment-resistant depression (TRD). The emergence of ketamine addresses this significant unmet clinical need. Its ability to significantly alleviate depressive symptoms within hours, particularly for patients with acute suicidal ideation, has made it a highly commercially viable option. Market analysis shows that ketamine's use in the antidepressant field is rapidly growing, and the global ketamine market is expected to reach RMB 13.33 billion by 2030, with the depression market dominating the market.
 Figure 1 Johnson & Johnson's Spravato nasal spray
Figure 1 Johnson & Johnson's Spravato nasal spray
Image source: Johnson & Johnson official website
2.1 Johnson & Johnson's Spravato: A pioneering market leader
In the commercialization of ketamine antidepressants, Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson, is undoubtedly a pioneer and leader in the market. Its esketamine hydrochloride nasal spray (trade name: Spravato, Chinese name: Su Kailang) is the first and currently the only ketamine-based drug approved by the U.S. Food and Drug Administration (FDA) and the China National Medical Products Administration (NMPA) for the treatment of depression. This first-mover advantage has secured it a commanding position in the market. Spravato's approval marks a new era in antidepressant treatment. Its rapid onset of action has revolutionized the traditional antidepressant treatment model, providing a new treatment option for patients with major depressive disorder (MDD) and treatment-resistant depression (TRD).
1) Sales in 2024 will exceed $1.1 billion, becoming a blockbuster drug
Since its approval in the United States in 2019, Spravato has seen tremendous market growth, demonstrating its immense commercial value. According to Johnson & Johnson's financial report, Spravato's global sales reached $689 million in 2023, an 84.22% year-on-year increase. By 2024, Spravato's annual sales were projected to reach $1.077 billion, officially joining the ranks of blockbuster drugs. According to Johnson & Johnson's 2025 interim report, its sales reached $734 million in the first half of the year, showing continued strong growth. Sales are projected to exceed $3 billion in 2027, potentially reaching a peak of $5 billion. This performance not only affirms its clinical value but also reflects the market's urgent need for fast-acting antidepressants. In China, with Spravato's official approval in April 2023, its market potential will be further unleashed. Considering China's large depression patient population and Spravato's significant efficacy advantages, its sales in the Chinese market are expected to experience explosive growth, becoming another key growth engine for Johnson & Johnson globally.
2) From adjuvant therapy to monotherapy, covering refractory depression (TRD)
Spravato's market success is not only due to its rapid onset of action, but is also closely related to its expanding indications. Initially, Spravato was approved for use in combination with an oral antidepressant for the treatment of treatment-resistant depression (TRD) in adults who have not responded adequately to at least two different antidepressants. On January 21, 2025, Johnson & Johnson announced that the FDA had approved Spravato's supplemental new drug application (sNDA), making it the first and only monotherapy for the treatment of treatment-resistant depression. The approval was based on a randomized, double-blind, multicenter, placebo-controlled pivotal study of Spravato, published in JAMA Psychiatry (IF=22.5). The results showed that Spravato alone demonstrated faster and superior improvement in the Montgomery-Asberg Depression Rating Scale (MADRS) total score compared to placebo. In a post-trial analysis, Spravato demonstrated numerical improvement in all 10 MADRS items at day 28. At week 4, 7.6% of patients taking placebo achieved remission (MADRS total score ≤ 12), compared with 22.5% of patients taking Spravato.
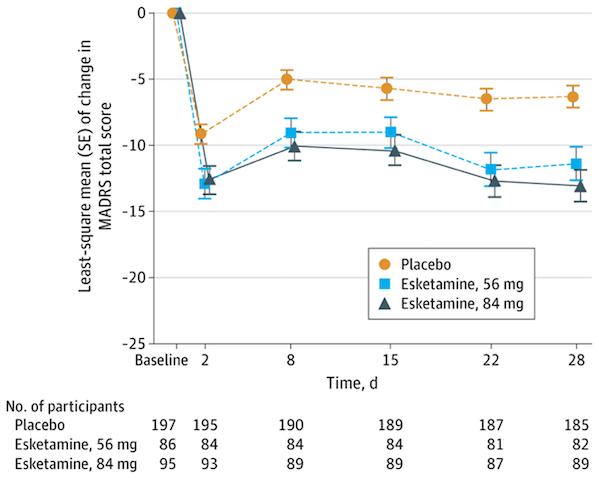 Figure 2 MADRS scores of patients in each group
Figure 2 MADRS scores of patients in each group
Image source: Reference 1
The continuous expansion of indications not only broadens the applicable population of Spravato, but also provides a continuous driving force for its market growth, enabling it to cover multiple treatment scenarios from refractory to acute phase, from combination therapy to monotherapy.
3) Dosage form advantages: Convenience and clinical acceptance of nasal spray dosage form
In terms of dosage form selection, the nasal spray dosage form used by Spravato is also one of the important factors for its market success. Compared with traditional intravenous injection or oral administration, nasal spray is non-invasive, convenient, and fast-acting. Patients can self-administer the drug under the supervision of medical staff in medical institutions. The whole process takes only a few minutes, which greatly shortens the treatment time and improves the patient's medical experience and treatment compliance. This method of administration is particularly suitable for patients in the acute phase who require rapid intervention, avoiding the inconvenience and pain caused by venipuncture. Professor Wang Gang, President of Beijing Anding Hospital, pointed out that compared with the intravenous use of esketamine injection, the nasal spray dosage form is innovative and has high patient satisfaction. Patients can leave the hospital after being observed for 2 hours after use in the emergency department, truly realizing rapid anti-suicidal treatment. This convenience not only improves the efficiency of clinical operations, but also reduces the operating costs of medical institutions, making it more easily accepted by clinicians and patients, thereby accelerating its promotion and application in the market.
2.2 Four domestic companies have entered the clinical stage of ketamine in the field of antidepressants
Faced with the huge success of Johnson & Johnson's Spravato in the global market and the huge potential of China's antidepressant drug market, domestic pharmaceutical companies have begun to make plans, and a research and development competition around ketamine and its isomers has begun.
Currently, competition in the domestic market primarily revolves around R-ketamine (dextrorotatory ketamine). Unlike S-ketamine (levorotatory ketamine), used in Johnson & Johnson's Spravato, R-ketamine is believed to have a better safety profile and fewer side effects. This differentiated R&D strategy provides domestic pharmaceutical companies with an opportunity to challenge Johnson & Johnson's market position.
Yaozhi data shows that among the many players, R-ketamine from Enhua Pharmaceutical, Hengrui Medicine, Purdue Pharmaceuticals and Renmin Pharmaceuticals has been approved to enter the clinical research stage, becoming the first tier in the domestic R&D competition landscape.
Judging from the institutions and PIs cooperating with each company in conducting clinical research, President Wang Gang of Beijing Anding Hospital is the company's main cooperating expert. The research results of Professor Wang Gang's team are highly scientific and innovative. Many of their research results have been published in top international medical journals and have been highly praised by international peers. Among them, the most representative study is a randomized controlled clinical trial published in JAMA Network Open in August 2023. The study aimed at patients with depression who were receiving adequate antidepressant treatment but whose condition fluctuated, and explored whether a single subanesthetic dose of esketamine intravenously could re-stimulate the efficacy of oral antidepressants. The results showed that compared with the control group, the treatment response rate of the esketamine treatment group was significantly higher, and this stimulatory effect could last for at least 6 weeks.
2.3 Global Research Progress on Different Dosage Forms of Ketamine
Clinical research on ketamine extends beyond the exploration of its efficacy and mechanisms and encompasses the development of various dosage forms and routes of administration. Different dosage forms have their own advantages and disadvantages in terms of speed of action, bioavailability, patient compliance, and safety, all of which are key considerations in dosage form design to prevent drug abuse. Therefore, developing diverse dosage forms is crucial to meeting diverse clinical needs and optimizing treatment options. Currently, clinical research focuses primarily on several dosage forms, including nasal sprays, intravenous injections, and oral sustained-release tablets.
Nasal sprays are currently the most intensively researched and widely used dosage form of ketamine antidepressants. Johnson & Johnson's Spravato (esketamine nasal spray) is a successful example of this dosage form, its convenient, non-invasive administration and rapid onset of action making it widely popular in clinical practice. Clinical research data from Spravato fully demonstrate its efficacy and safety in treating patients with refractory depression and depression accompanied by acute suicidal ideation. In China, in addition to the already marketed Spravato, companies such as Enhua Pharmaceutical, Hengrui Medicine, and Principal Pharma are also actively developing R-ketamine nasal sprays and have received approval for clinical trials.
The Yaozhi Consulting team has discovered that several companies around the world are developing new dosage forms of ketamine. Douglas Pharmaceuticals, a New Zealand-based pharmaceutical company, has completed a randomized, double-blind, placebo-controlled Phase 2 clinical study of R-107, a sustained-release ketamine formulation. (Clinical Trial Registration Number: ACTRN12618001042235) The results have been published in Nature Medicine (IF=50.0). The study enrolled 168 patients with TRD. At the 17-week follow-up, all treatment groups showed improvement in MADRS scores. The mean difference in MADRS scores between the 180mg dose group and the placebo group was -6.1 (p=0.019), meeting the clinical endpoint. Safety profile included no changes in blood pressure, minimal sedation, and mild dissociative symptoms (common adverse reactions with nasal sprays). The most common adverse reactions were headache, dizziness, and anxiety. The results demonstrate that R-107 sustained-release tablets are effective, safe, and well-tolerated in patients with TRD.
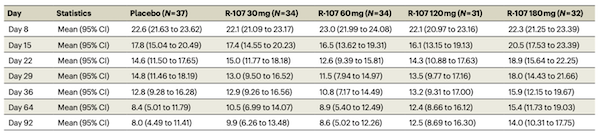 Figure 3 Mean changes in MADRS scores in each group at different follow-up times in the R-107 study
Figure 3 Mean changes in MADRS scores in each group at different follow-up times in the R-107 study
Image source: Reference 2
In terms of R-107 dosage form design, due to the use of polyethylene oxide, R-107 tablets are very hard and difficult to break, thus effectively preventing drug abuse. Therefore, during the randomization phase of the study, most patients completed the drug administration at home.
3. Commercialization Challenges: Regulation, Payment, and Abuse Risks
Despite ketamine's enormous market potential in the antidepressant field, its path to commercialization has been challenging. As a substance with hallucinogenic and addictive potential, ketamine's transformation from a "party drug" to a "miracle antidepressant" has been accompanied by a series of daunting challenges.
These challenges primarily focus on regulation, payment, and abuse risk, collectively constituting hurdles that must be overcome in the commercialization of ketamine-based drugs. Balancing the significant therapeutic benefits with potential risks is a common challenge faced by regulators, medical institutions, pharmaceutical companies, and patients.
3.1 Strict Control of Ketamine as a Category I Psychotropic Drug
Ketamine's regulatory risks are the primary challenge facing its commercialization. Due to its well-established abuse potential and hallucinogenic effects, ketamine is subject to strict legal regulations worldwide. In China, ketamine is classified as a Class I psychotropic drug, and its production, sale, transportation, and use are strictly regulated by the State Food and Drug Administration. This means that any medication containing ketamine or its isomers must adhere to more stringent regulatory procedures than those for standard prescription drugs. For example, Spravato must be administered in a certified medical institution under the full supervision of professional medical staff, and patients must remain under observation for at least two hours after administration. While this strict regulatory model maximizes drug safety, it also significantly limits its accessibility. Medical institutions must invest additional resources to meet regulatory requirements, which undoubtedly increases their operating costs and may discourage some from using the drug, thus hindering its widespread adoption.
3.2 High treatment costs and medical insurance coverage issues
High treatment costs are another major challenge facing the commercialization of ketamine-based drugs. Johnson & Johnson's Spravato, for example, is priced between $545 and $800 per treatment in the US. Based on the dosage indicated in the drug package insert, the initial monthly treatment cost is between $4,720 and $6,785, with maintenance costs ranging from approximately $2,360 to $3,540 per month. Despite its relatively low price in China, at 798 yuan per 28mg vial, the drug is not currently covered by medical insurance, making it a significant expense for the average patient. Globally, national medical insurance systems are still exploring and developing policies to cover ketamine for antidepressant treatment. In China, Spravato is not yet included in the national medical insurance catalogue, meaning patients must cover the entire cost of treatment out-of-pocket. This represents a significant financial burden for a mental illness requiring long-term treatment. The high cost not only limits patient use but may also lead some to seek informal, less expensive "off-label" treatments, which poses greater safety risks. Therefore, how to improve the affordability of drugs through reasonable pricing strategies and active medical insurance negotiations is a problem that pharmaceutical companies and the government need to solve together.
3.3 How to avoid drug abuse
The abuse risk of ketamine is the most sensitive and concerning issue during its commercialization. Before becoming an antidepressant, ketamine was more widely known as "K powder," a recreational drug abused in venues. Its abuse can lead to a range of serious health problems, including cognitive impairment, urinary tract damage, and even death. Therefore, when ketamine was reintroduced as a pharmaceutical, public and regulatory concerns about its abuse risk were understandable. While the dosage, administration, and regulation of ketamine for medical use differ significantly from those for abuse, its potential for addiction remains an unavoidable concern. To minimize the risk of abuse, countries have implemented extremely strict regulatory measures to ensure that the drug is used only under medical supervision. However, clearly communicating the distinction between medical use and illegal abuse to the public, alleviating unnecessary panic, and establishing a comprehensive monitoring and early warning system to prevent the drug from diverting from medical channels into the illicit market will be a long and arduous task.
IV. Conclusion
Ketamine's history is filled with dramatic twists and turns. It's both a life-saving drug and a life-destroying drug. This dual identity presents significant ethical and regulatory challenges in its medical use. On the one hand, it offers new hope to countless patients struggling with traditional antidepressant treatments; on the other, its history of abuse and potential risks have made it a subject of considerable controversy.
Establishing a regulatory system that ensures safe and effective patient access to treatment while strictly preventing drug abuse and illicit distribution is a common challenge facing governments and medical institutions worldwide. From "party drug" to "miracle antidepressant," ketamine's saga continues, and how to write its future will test the wisdom and conscience of all humanity.
https://news.yaozh.com/archive/46067.html
By editorRead more on
- Lunan Pharmaceutical’s Jiali® Oxaliplatin Injection Receives US Marketing Approval January 19, 2026
- The second NDA for gumozymab, a new drug application for the treatment of ankylosing spondylitis, has been accepted January 19, 2026
- The clinical trial application for its shingles mRNA vaccine has been officially accepted January 19, 2026
- 3.1 billion! Orthopedic giant announces major acquisition. January 19, 2026
- The Small Nucleic Acid Track is Ushering in a Major Explosion January 19, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



