New therapy breaks the deadlock in treating the “king of gynecological cancer”
July 31, 2025
Source: drugdu
 301
301
Recently, Tongyi Pharmaceutical's first dual-ligand small drug conjugate CBP-1008 (Rico-V, Ruikefutai) received approval from the NMPA to conduct a Phase III clinical trial in platinum-resistant ovarian cancer (PROC registration).
The drug is reportedly a fully chemically synthesized, small bi-ligand drug conjugate (Bi-XDC) with a molecular weight approximately 1/50 of that of traditional ADCs, offering unique advantages in terms of CMC and cost. Rico-V has been approved for Phase III clinical trials and is expected to become the world's first Bi-XDC drug, offering a new treatment option for ovarian cancer patients.
New therapy breaks the deadlock in treating the "king of gynecological cancer"
Ovarian cancer ranks first in mortality among gynecological malignancies, earning it the nickname "the king of gynecological cancers." Because it lacks specific early symptoms and is often diagnosed in its advanced stages, it's also known as the "silent killer."
According to statistics from the National Cancer Center in 2024, my country has approximately 61,100 new cases of ovarian cancer and 32,600 deaths annually. Approximately 70% of patients are diagnosed at an advanced stage. The current mainstay of treatment is surgery combined with platinum-based chemotherapy and maintenance therapy with targeted drugs, but most patients will relapse and develop platinum resistance. Patients with platinum-resistant ovarian cancer (PROC) have a poor prognosis and extremely limited treatment options.
Although some innovative drugs have been approved recently, such as AbbVie's FRαADC drug ELAHERE® (somituximab injection), the treatment needs of PROC patients are far from being met.
CBP-1008 offers hope for this difficult situation. Developed based on Tongyi Pharmaceutical's proprietary Bi-XDC technology platform, it is the world's first dual-ligand drug conjugate that can simultaneously target both FRα and TRPV6 receptors expressed on tumor cell membranes.
According to clinical data previously released by Tongyi Pharmaceutical, CBP-1008 demonstrated significant efficacy in patients with platinum-resistant OC (PROC) who had previously received 1-3 lines of therapy, with superior overall survival (OS) compared to chemotherapy and ADCs. The median OS reached 19.4 months (N=163), surpassing the historical average OS of 10.9-13.9 months for single-agent chemotherapy (≤3 lines of PROC) and 15.6-16.5 months for sometuximab. Furthermore, CBP-1008 demonstrated efficacy in patients with low folate receptor expression, regardless of folate expression levels.
In terms of safety, most adverse events (AEs) were mild to moderate. Furthermore, compared with other antibody-drug conjugates (ADCs) using monomethyl auristatin E (MMAE) as a drug carrier, Rico-V showed no significant ocular toxicity or peripheral neurotoxicity.
In terms of production cost and drug supply, Rico-V is a small dual-target conjugate drug that is fully chemically synthesized. Its molecular weight is approximately 1/50 of that of traditional ADC drugs. In terms of CMC, it has the advantages of simple synthesis/preparation, defined compound structure, and easy control of production processes and quality indicators. Therefore, its production cost and drug expenses are much lower than those of ADC drugs.
The NMPA approval of CBP-1008 for a Phase III clinical trial in platinum-resistant ovarian cancer (PROC registration) marks an important step towards clinical application and is expected to provide a new treatment method for patients with platinum-resistant ovarian cancer.
Bi-XDC: The next generation of coupling star
Traditional targeted chemotherapy drugs in the clinical treatment of cancer suffer from shortcomings such as low targeting, significant side effects, the development of drug resistance, and high recurrence rates. ADCs (antibody-drug conjugates) combine traditional chemotherapy drugs with recombinant monoclonal antibody molecules via a linker, giving these drugs active targeting capabilities and enabling precision cancer treatment.
However, ADC drugs are often biological drugs with large molecular weight, and they face difficulties such as complex production processes, high drug costs, and poor clinical safety.
Tongyi Pharmaceutical has developed a new technology - bi-ligand drug conjugate (Bi-XDC), which has opened up a new path for drug development.
The Bi-XDC drug molecule consists of four components: ligand A targeting receptor 1, ligand B targeting receptor 2, a therapeutic payload, and a linker. Bi-XDC precisely delivers the coupled payload to specific cells through the synergistic interaction of the two ligands with surface receptors targeting tumor cells.
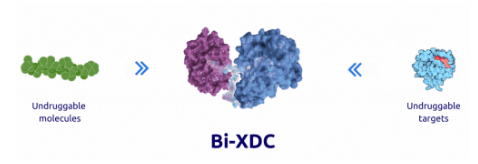
Compared to traditional ADCs, Bi-XDC drugs offer numerous advantages, including a richer target population, reduced toxicity, and low cost (it can be fully chemically synthesized). Firstly, Bi-XDC utilizes dual ligand pairing to effectively enhance target specificity and affinity, transforming two previously undruggable single targets into a druggable dual-target pair, thereby enabling the targeting of a wider range of undruggable targets. Secondly, Bi-XDC maintains a relatively small molecular weight, approximately 1/50 that of ADC drugs, enabling faster cell entry and faster clearance from non-lesional tissues, significantly improving efficacy and safety.
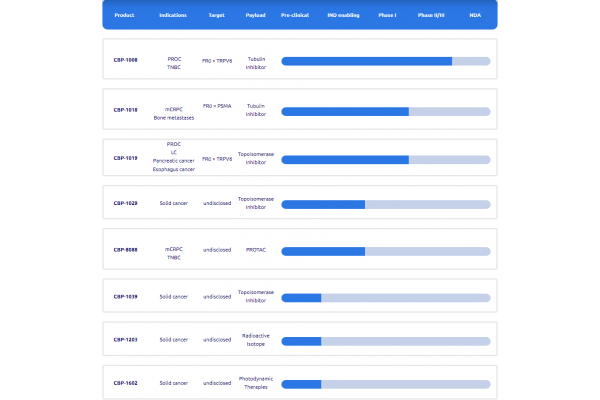
Currently, Tongyi Pharmaceutical has three Bi-XDC products entering the clinical stage, namely CBP-1008, CBP-1018 and CBP-1019, covering high-demand areas such as ovarian cancer, prostate cancer, and endometrial cancer.
Small molecules (such as folic acid, biotin, lactic acid, glycyrrhetinic acid, etc.) are easy to obtain, inexpensive, low in toxicity, have no immune stress response, and are easy to characterize and modify. They are often used as targeting groups for anticancer drugs and are widely used in active targeted therapy.
Among them, folic acid (FA) is a high-affinity ligand for the folate receptor, allowing for simple coupling with other ligands, making it considered an excellent targeting agent. The folate receptor is highly expressed in a variety of tumor cells, including those of the brain, breast, cervix, colon, epithelium, kidney, and lung, as well as over 90% of ovarian cancer cells. It is non-toxic, stable, and easy to bind and characterize. However, it is barely expressed in normal tissues, making it a popular target for drug development in tumors such as ovarian cancer.
Tongyi Pharmaceutical's three products that have entered the clinical stage are all small molecule FRα dual-ligand conjugated drugs.
CBP-1008 targets folate receptor α (FRα) and transient receptor potential vanilloid receptor 6 (TRPV6) using MMAE as a toxin. It is Tongyi Pharmaceutical's first Bi-XDC technology product. Currently, the drug has received approval from the National Medical Products Administration (NMPA) to conduct a Phase III clinical trial in platinum-resistant ovarian cancer (PROC registration).
CBP-1018 targets FRα and PSMA, and its toxin is MMAE. Initial efficacy has been observed in patients with mCRPC, further validating the Bi-XDC platform.
According to data presented by Tongyi Pharmaceutical at the 2024 ASCO, 52% of patients receiving CBP-1018 monotherapy at doses of 0.14 mg/kg and above experienced a decrease in PSA levels, including 9 patients with measurable lesions. The objective response rate (ORR) and disease control rate (DCR) reached 33.3% (3/9) and 100% (9/9), respectively. Among all evaluable patients with mCRPC, the median radiographic progression-free survival (rPFS) was 9.2 months. CBP-1018 was well tolerated, with the MTD not reached. Currently, the FDA has approved a Phase I/II clinical trial application for CBP-1018 in combination with a novel endocrine therapy (enzalutamide).
CBP-1019 is a next-generation FRα and TRPV6 dual-ligand drug conjugate, loaded with DX-8951, a camptothecin derivative from the topoisomerase I inhibitor (TOPOIi) class. The drug has received Orphan Drug Designation from the US FDA for three indications: pancreatic cancer, esophageal cancer, and small cell lung cancer, as well as Fast Track Designation for the treatment of recurrent endometrial cancer (EC) following at least one line of systemic platinum-based chemotherapy.
Preliminary data from its Phase I/II clinical trial showed that CBP-1019 demonstrated excellent efficacy in 10 patients with advanced/metastatic EC treated with either 3.0 mg/kg or 4.0 mg/kg doses, regardless of FRα/TRPV6 expression status. At the 3.0 mg/kg dose level (potentially recommended Phase II dose, RP2D), seven of the nine patients with advanced/metastatic EC had at least one post-dose tumor assessment, achieving an objective response rate (ORR) of 42.9% and a disease control rate (DCR) of 100%. Furthermore, CBP-1019 demonstrated a favorable safety and tolerability profile, with no observed adverse events typical of TOPO II-based ADCs, such as interstitial lung disease (ILD), stomatitis, and ocular toxicity.
Notably, the Bi-XDC technology platform can theoretically deliver a variety of payloads, including small molecule toxins, PROTACs, radioisotopes, photodynamic therapy, immunomodulators, NK cells, and T cells. Tongyi Pharmaceutical has already developed cutting-edge approaches such as PROTAC Bi-XDC and radioligand Bi-XDC, expanding dual-targeting technology into a broader therapeutic area. However, these therapies are still in the preclinical stage, and their safety and efficacy remain to be verified.
Conclusion
Tongyi Pharmaceuticals, with its unique Bi-XDC technology platform, has secured approval for Phase III clinical trials for its first novel drug, CBP-1008. However, first-in-class innovative drugs present both risks and opportunities. Bringing novel mechanisms of action to market carries the risk of failure at every stage. If CBP-1008 succeeds in Phase III trials, it will offer a breakthrough treatment option for patients with platinum-resistant ovarian cancer, establishing Tongyi Pharmaceutical as a leader in this field.
Source:https://news.yaozh.com/archive/45835.html
By editorRead more on
- Lunan Pharmaceutical’s Jiali® Oxaliplatin Injection Receives US Marketing Approval January 19, 2026
- The second NDA for gumozymab, a new drug application for the treatment of ankylosing spondylitis, has been accepted January 19, 2026
- The clinical trial application for its shingles mRNA vaccine has been officially accepted January 19, 2026
- 3.1 billion! Orthopedic giant announces major acquisition. January 19, 2026
- The Small Nucleic Acid Track is Ushering in a Major Explosion January 19, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



