collision of targets and technologies
February 21, 2025
Source: drugdu
 355
355
In 1982, Weinberg and other laboratories discovered HRAS in human bladder cancer cells T24/EJ, making RAS the first human tumor gene discovered. Subsequently, other tumor genes KRAS and NRAS were also discovered.
However, in the following decades, no targeted drugs for the KRAS gene have been successfully developed, and KRAS is considered an "undruggable" target. In 2021, the first KRAS G12C inhibitor, Sotorasib, was launched, making KRAS a hot target in the field of oncology. However, its clinical and commercialization progress after its launch was not satisfactory.
Therefore, pan-KRAS inhibitors that can target multiple mutation forms of the KRAS gene have become a new research direction in the field of KRAS targeted drugs.
The close connection between KRAS gene and cancer
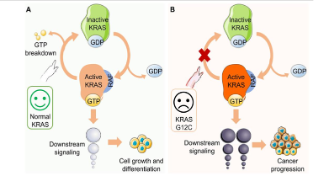
The full name of the KRAS gene is Kirsten Rat Sarcoma Viral Oncogene Homolog, which means "Kirsten rat sarcoma viral oncogene homolog" in Chinese. It is a proto-oncogene that encodes a small GTPase transduction protein called KRAS.
KRAS protein is part of the RAS/MAPK pathway, which transmits signals from outside the cell to the cell nucleus. These signals instruct cells to grow and divide or mature and assume special functions. It plays a core role in the key signaling pathways that regulate cell proliferation, differentiation and survival.
KRAS carcinogenesis
Under normal circumstances, KRAS protein transmits signals by cyclically switching between binding to GTP (activated state) and GDP (inactive state); however, when KRAS undergoes oncogenic mutations, its GTPase activity is lost, causing the protein to remain in an activated state, driving uncontrolled cell growth and tumorigenesis.
The KRAS gene is a core member of the RAS oncogene family. Its abnormal function directly drives tumorigenesis and is one of the most commonly mutated genes in cancer. Studies have shown that KRAS gene mutations are associated with up to 20% to 30% of human cancers. In human cancers, KRAS gene mutations occur in nearly 70% of pancreatic cancers, 30-50% of colon cancers, 17% of endometrial cancers, and 20% of lung cancers. It also occurs in cancer types such as bile duct cancer, cervical cancer, and melanoma.
KRAS mutation types
Among the KRAS gene mutations, 97% are mutations in the 12th or 13th amino acid residues, among which the most important are G12D, G12V, and G13D.
When KRAS undergoes G12D, G12V, or G13D mutations, the activity of GAP will be destroyed, and KRAS will remain bound to GTP, locking KRAS in an active tyrosine kinase state and continuously activating downstream signaling pathways (such as PI3K, RAF-MEK-ERK, RAL-GEF, etc.).
After the downstream signaling pathway is turned on, it stimulates cell proliferation and migration, eventually forming malignant tumors.
KRAS resistance mechanism
KRAS mutations may be associated with resistance to chemotherapy and EGFR targeted therapy. A 2021 study showed that KRAS amplification may be the cause of primary resistance to anti-EGFR monoclonal antibodies in patients with colorectal cancer. Since then, several studies have reported that KRAS G12A, G12C, and G12D mutations can mediate resistance, which is seen in 2% to 7% of resistant cases.
The history and challenges of “undruggable” targets
Due to its close connection with cancer, KRAS has long been regarded as a "core target" for cancer treatment. However, due to its featureless, nearly spherical structure, KRAS lacks binding sites suitable for small molecule drugs, and it is difficult to design specific inhibitors using the traditional method of inhibiting active sites, making KRAS regarded as an "untargetable" drug target for more than 30 years after its discovery.
Difficulties in drug development for KRAS targets
The currently known active functional domains of KRAS are mainly pocket-shaped functional domains where KRAS binds to GDP or GTP. KRAS binds very strongly to the substrate GTP, with an affinity coefficient of picomolar (10-12) levels, while the concentration of GTP in normal cells is micromolar (10-6) levels. At the same time, the surface of the KRAS protein is smooth and lacks obvious drug binding pockets, making it difficult for traditional small molecule drugs to bind to it. Therefore, it is difficult to block the activity of the KRAS protein through competitive inhibitors, making it difficult to develop nucleotide competitive inhibitors that directly target the GTP pocket.
In addition, there are many types of KRAS mutations, and it is challenging to develop drugs for different mutations. At the same time, the normal activity of KRAS is also the activity required for many normal cell functions. Small molecules targeting the active site of mutant KRAS can often inhibit the activity of wild-type KRAS. If drugs that directly inhibit KRAS are developed, the drug toxicity and side effects will be greater. KRAS has a high homology with NRAS and HRAS. Drugs that can inhibit the activity of KRAS are likely to inhibit the activity of NRAS and HRAS.
Commercialization of KRAS inhibitors is hindered
In 2018, Wellspring Biosciences published preclinical data of the KRAS G12C inhibitor ARS-1620 in Cell, triggering the first large-scale KRAS research and development boom. Significant breakthroughs have been made in the research and development of KRAS inhibitors, especially the development of inhibitors targeting specific mutation sites (e.g., G12C and G12D).
In 2021, the first KRAS G12C inhibitor Sotorasib was approved for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) carrying the KRAS p.G12C mutation who have previously received at least one systemic treatment, marking a new stage in KRAS targeted therapy.
To date, four KRAS inhibitors have been approved for marketing worldwide, all targeting KRAS G12C. According to incomplete statistics, there are approximately 70 research projects targeting KRAS worldwide, and more than half of the trials are conducted around KRAS G12C inhibitors.
However, the commercialization performance of KRAS G12C inhibitor products after launch was poor.
In the three years after Sotorasib was launched in 2021, its sales were US$90 million, US$285 million, and US$280 million, respectively. In addition to the impact of failing to convince the US FDA to change Sotorasib from accelerated approval to full approval in October 2023, its own limitations are also a factor that hinders the commercialization of KRAS G12C inhibitor products.
In addition, KRAS G12C inhibitors are only suitable for patients with G12C mutations, but not for patients with other mutations or wild types. The coverage population is limited, which makes the range of approved indications narrow. At the same time, some patients will develop new mutations such as KRAS G12D and G12V after taking the drug for a period of time, or bypass KRAS G12C inhibition through upstream RTK signal activation, leading to drug resistance.
Research and development progress of pan-KRAS inhibitors
Pan-KRAS inhibitors are a new class of targeted anticancer drugs that recognize the common structural features of the KRAS protein (such as the Switch-II pocket) and widely inhibit various mutation forms including G12C, G12D, and G13D through covalent or non-covalent binding. They are expected to break through the limitations of current KRAS targeted drugs in terms of coverage and drug resistance, and are considered to be the key direction for conquering KRAS-driven cancers.
Studies have shown that 16.6% of cancer patients in China carry KRAS mutations, of which KRAS G12C accounts for only 14.5% of all KRAS mutation. Pan-KRAS covers more KRAS mutation types, including G12D and G12V, and its clinical value will be higher than that of KRAS G12C, which targets a single mutation.
Drug resistance is one of the main challenges currently faced by KRAS G12C inhibitors, and pan-KRAS inhibitors are expected to prolong the treatment cycle of patients with KRAS G12C resistance. For example, in clinical plans, mutant-selective inhibitors are first used in KRAS mutants, and pan-KRAS inhibitors are selected after drug resistance is generated, thereby obtaining additional clinical benefits in the later stage. In addition, pan-KRAS inhibitors can prevent or delay compensatory activation between RAS mutations, reduce the occurrence of drug resistance, and improve treatment effects.
The research and development of pan-KRAS inhibitors is still in its early stages. There are currently a number of pan-KRAS inhibitors under development worldwide, but all are in the preclinical or early clinical stages.
Future prospects of pan-KRAS inhibitors
PROTACs and molecular glue are two targeted protein degradation technologies that have developed rapidly in recent years. They achieve protein degradation in disease treatment through different mechanisms and have the potential to change the traditional way of drug development. Currently, many companies around the world have used PROTAC or molecular glue technology to develop pan-KRAS inhibitors. On the other hand, quantum computing combined with artificial intelligence has been used to discover candidate molecules for pan-KRAS inhibitors.
Molecular glue
Revolution Medicines in the United States has established a "ternary complex inhibitor" research and development platform and developed a series of molecular glue-type macrocyclic peptidomimetic inhibitors. Among them, RMC-6236 is a pan-RAS-on non-covalent inhibitor developed by Revolution Medicines based on the mechanism of action of molecular glue, and has now entered Phase I clinical trials. RMC-6236 can bind to the chaperone protein cyclophilin A (CYPA), and the formed binary complex can bind to different RAS (ON) proteins, including three oncogenic mutant proteins with the most common mutation sites (G12, G13 and Q61), thereby forming a three-complex - CYPA: drug: KRAS, thereby disrupting oncogenic signal transduction and tumor growth.
PROTACs
Protein degraders represented by PROTAC are considered to be new opportunities for targeting traditionally undruggable proteins. According to incomplete statistics, there are about 20 PROTAC drugs targeting KRAS under development worldwide, and the fastest-progressing ones have entered Phase I clinical trials. Most drugs target a KRAS mutant. A 2024 study demonstrated a small molecule PROTAC selective pan-KRAS degrader called ACBI3, which was shown to be able to efficiently and selectively degrade 13 of the 17 most common KRAS mutants. Targeted PROTACs are well tolerated and lead to tumor regression. The study believes that these results reveal a new way to treat KRAS-driven cancers with PROTAC degraders.
Quantum computing and artificial intelligence
Technologies such as quantum computing and artificial intelligence show the potential to transform the drug discovery process. In a study in January 2025, in order to generate potential new KRAS inhibitors, researchers combined quantum computing with classical computing methods and proposed a quantum-classical hybrid framework model that combines a quantum variational generative model (QCBM) and a long short-term memory network (LSTM). After using the hybrid model to generate 1 million candidate molecules, the research team applied an artificial intelligence engine to filter them and ultimately identified 15 candidate molecules. Among them, ISM061-018-2 showed a superior potency in binding to the target protein than other molecules. At the same time, this molecule did not show significant nonspecific cytotoxicity even at high concentrations. It also showed dose-dependent inhibitory activity against five other common mutant KRAS as well as wild-type HRAS and NRAS, demonstrating its potential as a pan-KRAS inhibitor with a novel structure.
KRAS is one of the most common mutated genes in cancer and is regarded as a core target for cancer treatment, but due to limitations such as structure, it has long been regarded as an "undruggable" target. The approval of the KRAS G12C inhibitor marks a new stage in KRAS targeted therapy, but its clinical and commercialization progress has been unsatisfactory. Therefore, pan-KRAS inhibitors are regarded as a new breakthrough for KRAS targeted therapeutics due to their advantages in terms of patient coverage, and many pipelines around the world have entered the early clinical stage. In the future, molecular glue, PROTAC technology, quantum computing and artificial intelligence will accelerate the research and development of pan-KRAS inhibitors and bring lasting clinical benefits to patients with KRAS-driven cancers.
https://news.yaozh.com/archive/45008.html
By editorRead more on
- Phase III clinical trial of vetcotozumab completes patient enrollment February 9, 2026
- The first long-acting coagulation factor VIII, has officially entered the Chinese mainland market. February 9, 2026
- 17.9 billion yuan! A top-selling topical medication emerges. February 9, 2026
- Turnaround! Generic drug giant successfully “revived” February 9, 2026
- Novartis’s first-ever autoimmune drug has been submitted for marketing approval in China. February 9, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



