The mechanism by which G protein mutations lead to excessive activation of T cells through non classical pathways
October 23, 2024
Source: drugdu
 328
328
Writing | Snow Moon
G protein coupled receptors (GPCRs) guide cellular responses to environmental signals, including hormones, neurotransmitters, and chemokines. This process is achieved through a complex biochemical cycle mediated by heterotrimeric G protein. After GPCR binds to the ligand, the G α subunit binds to GTP, becomes active, and separates from the G β - γ complex and GPCR. The separated G α - guanosine triphosphate (GTP) and free G β - γ initiate downstream signaling, including the generation of second messengers, biochemical interactions, and ion channel changes. The GTPase activity of G α hydrolyzes GTP to guanosine diphosphate (GDP), thereby terminating signal transduction and allowing Gabg to reassemble with GPCRs, enabling cells to respond to reactivation of GPCRs. In the G α protein family, the Gai/o family includes inhibitory subtypes that regulate biological responses by inhibiting the production of cyclic adenosine monophosphate (cAMP) by adenyl cyclase AC. AC is widely expressed as a transmembrane isomer, with different tissues expressing multiple isoforms at different levels, but only certain ACs are sensitive to the inhibitory effect of Gai. Gai2 (encoded by GNAI2) has been recognized to play a role in the normal functioning of the cardiovascular, nervous, endocrine, and immune systems. The G α i2 protein belongs to the heterotrimeric G protein, which inhibits cAMP production by regulating adenylate cyclase. However, the specific role of Gai2 in human physiology and development, including the impact of germline GNAI2 mutations in humans, is still unclear. Furthermore, it is not fully understood how active Gai/o regulates downstream signal transduction in the proximal domain through alternative mechanisms that do not involve adenylate cyclase mediated cAMP generation.
Helen C. Su's team from the National Institutes of Health in the United States published an article titled "Germline mutations in a G protein identifying signaling cross-talk in T cells" in Science. This article identified some patients carrying previously unreported or extremely rare GNAI2 mutations to study their clinical manifestations and potential disease mechanisms. When analyzing the molecular mechanisms by which these mutations significantly affect the immune system, the authors validated the hypothesis that Gai2 can regulate immune cell function without relying on cAMP. The team identified a total of 20 patients from different families carrying rare or unreported mutations in the GNAI2 gene (encoding the G α i2 protein) through whole exome and whole genome sequencing. The author also verified the functional changes of the mutant protein through biochemical methods. These mutations enhance the binding ability of G α i2 protein to GTP and decrease the hydrolysis rate of GTP, indicating that the mutated G α i2 protein is in a sustained active state.
The team identified a total of 20 patients from different families carrying rare or unreported mutations in the GNAI2 gene (encoding the G α i2 protein) through whole exome and whole genome sequencing. The author also verified the functional changes of the mutant protein through biochemical methods. These mutations enhance the binding ability of G α i2 protein to GTP and decrease the hydrolysis rate of GTP, indicating that the mutated G α i2 protein is in a sustained active state.
Through in vitro experiments, researchers found that the mutated G α i2 protein has a stronger inhibitory effect on cAMP, affecting the migration ability of immune cells (T cells and neutrophils). This may be due to the disruption of GPCR signaling, which prevents immune cells from effectively responding to chemokines and makes patients more susceptible to infection.
In addition, the author detected that the mutated G α i2 can also regulate T cell activation and proliferation through the RASA2 protein in the RAS protein family. The patient's T cells exhibit enhanced activation response, especially abnormal proliferation after T cell receptor stimulation. This phenomenon is caused by the enhancement of the RAS-MAPK and PI3K-AKT signaling pathways, rather than the classical cAMP pathway.
Finally, the patient's entire body was tested, and the results showed that the impact of GNAI2 mutations is not limited to the immune system, but also involves developmental defects in multiple organs. The patient exhibits various developmental abnormalities, such as developmental problems in the brain, bones, and endocrine system.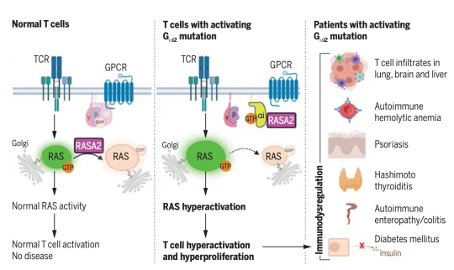 The summary indicates that GNAI2 mutations regulate T cell function through cAMP independent pathways, particularly the RAS pathway, and trigger autoimmune and multi organ dysfunction. This study defines a novel immune regulatory mechanism, explains how G α i2 mutations trigger T cell overactivation through the RAS signaling pathway, and provides new insights for the development of therapeutic strategies for immune related diseases in the future.
The summary indicates that GNAI2 mutations regulate T cell function through cAMP independent pathways, particularly the RAS pathway, and trigger autoimmune and multi organ dysfunction. This study defines a novel immune regulatory mechanism, explains how G α i2 mutations trigger T cell overactivation through the RAS signaling pathway, and provides new insights for the development of therapeutic strategies for immune related diseases in the future.
Read more on
- The first subject has been dosed in the Phase I clinical trial of Yuandong Bio’s EP-0210 monoclonal antibody injection. February 10, 2026
- Clinical trial of recombinant herpes zoster ZFA01 adjuvant vaccine (CHO cells) approved February 10, 2026
- Heyu Pharmaceuticals’ FGFR4 inhibitor ipagoglottinib has received Fast Track designation from the FDA for the treatment of advanced HCC patients with FGF19 overexpression who have been treated with ICIs and mTKIs. February 10, 2026
- Sanofi’s “Rilzabrutinib” has been recognized as a Breakthrough Therapy in the United States and an Orphan Drug in Japan, and has applied for marketing approval in China. February 10, 2026
- Domestically developed blockbuster ADC approved for new indication February 10, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



