Nature Journal | Revealing the Cell Membrane Protein Interactions of Endogenous GLP-1 Receptors!
September 12, 2024
Source: drugdu
 282
282
As a drug therapy target for obesity and type 2 diabetes, glucagon like peptide-1 receptor (GLP-1R) is widely distributed in many tissues and has cell type specific physiological functions. For example, GLP-1R mainly regulates insulin secretion and cell proliferation in pancreatic islets, and has functions such as appetite suppression, neuroprotection, and anti-inflammatory in the brain.
Considering that the maturation, localization, activation, and endocytosis of receptors are all influenced by protein interactions, exploring the interactions between receptors and other membrane proteins on the cell surface can provide important clues for the study of the regulatory mechanism of GLP-1R.
However, due to the low expression levels and transient and weak interactions of most membrane receptors, the study of the interaction between endogenous GLP-1R and cell membrane proteins requires the development of new research methods.
On September 3, 2024, the iHuman Institute of ShanghaiTech University and the research group led by Shui Wenqing from the School of Life Sciences and Technology, in collaboration with the research group led by Zhuang Min, published a research paper titled "Endogenous cell membrane interactome mapping for the GLP-1 receptor in different cell types" in the journal Nature Chemical Biology.
This study developed ligand based proximity labeling technology to achieve specific labeling, omics identification, and dynamic regulation analysis of the interacting proteome of endogenous GLP-1R expression.
The research team first designed two probes coupled with GLP-1 ligands and neighboring labeled enzymes, predicting that the probes could label neighboring cell membrane proteins that interact with receptors after binding and activating them (Figure 1).
In 293T cells overexpressing receptors and two types of cells expressing endogenous receptors (INS-1E pancreatic beta cells and SK-N-SH neurons), researchers have demonstrated through a series of validation experiments that the probe coupled with ascorbate peroxidase 2 (APEX2) has activity comparable to natural GLP-1 in binding receptors, activating downstream signals, and inducing endocytosis, and can achieve highly specific cell surface labeling.
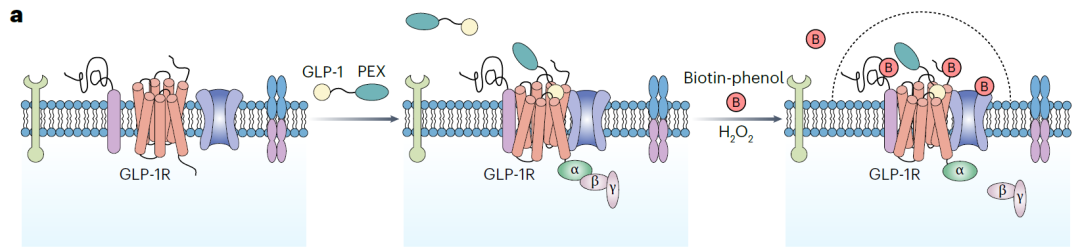
Figure 1: Experimental design based on ligand based proximity labeling
Then, the research team combined neighboring markers with quantitative proteomics techniques to analyze the interaction groups of endogenous GLP-1R membrane proteins in INS-1E pancreatic beta cells and SK-N-SH neurons, respectively.
It is interesting that the interaction groups obtained from these two types of cells are completely independent in molecular composition and have low overlap with potential interaction proteins overexpressing GLP-1R recorded in the database, reflecting the uniqueness of the interaction proteome of endogenous receptors in different cell types.
The research team first validated the interaction between 11 candidate proteins and GLP-1R using biochemical experiments for several newly discovered potential interacting proteins in two types of cells. Secondly, in INS-1E β cells, it has been demonstrated that knocking down five interacting proteins significantly enhances the insulin release effect stimulated by GLP-1 under high glucose conditions.
Further mechanistic studies suggest that these cell membrane interacting proteins primarily weaken GLP-1 stimulated insulin release by inhibiting the GLP-1 R-mediated cAMP signaling pathway.
Based on the comprehensive functional experiment results, this study ultimately discovered three previously unknown GLP-1R regulatory factors located on the cell membrane - SLC4A4, ATP1B3, and GPC4. Finally, the research team conducted time-resolved neighbor marker omics experiments in INS-1E β cells, tracking changes in membrane protein interactions during receptor activation and endocytosis at multiple time points, and constructing a dynamic cell membrane protein interaction profile of endogenous GLP-1R (Figure 2).
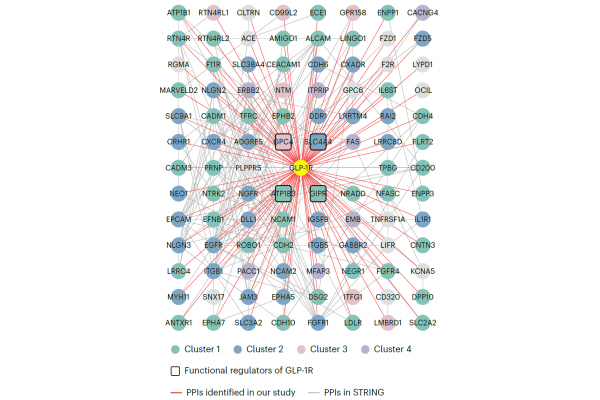
Figure 2: Cell membrane protein interaction profile of endogenous GLP-1R in INS-1E cells
In summary, this study not only provides a novel chemical biology technique for the analysis of the interaction between endogenous GLP-1R cell membrane proteins, but also lays the foundation for a deeper understanding of its cell type specific functional regulatory mechanisms.
GLP-1R belongs to the G protein coupled receptor family, and this ligand based proximity labeling strategy is expected to be extended to other important drug targets in this family, providing new theoretical basis for studying tissue-specific drug mechanisms of action.
Paper link: https://www.nature.com/articles/s41589-024-01714-1
By editorRead more on
- The first subject has been dosed in the Phase I clinical trial of Yuandong Bio’s EP-0210 monoclonal antibody injection. February 10, 2026
- Clinical trial of recombinant herpes zoster ZFA01 adjuvant vaccine (CHO cells) approved February 10, 2026
- Heyu Pharmaceuticals’ FGFR4 inhibitor ipagoglottinib has received Fast Track designation from the FDA for the treatment of advanced HCC patients with FGF19 overexpression who have been treated with ICIs and mTKIs. February 10, 2026
- Sanofi’s “Rilzabrutinib” has been recognized as a Breakthrough Therapy in the United States and an Orphan Drug in Japan, and has applied for marketing approval in China. February 10, 2026
- Domestically developed blockbuster ADC approved for new indication February 10, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



