FDA clears Braeburn’s long-acting Brixadi to treat opioid use disorder
May 26, 2023
Source: drugdu
 254
254
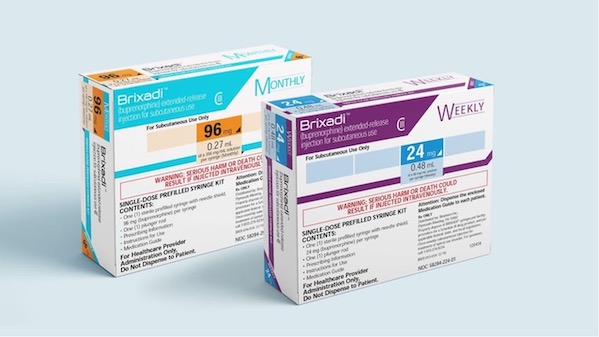
As the United States' opioid epidemic rages on, health officials are busy looking for ways to help with the response.
Now, right on the heels of the FDA's approval for a new overdose rescue medication, the agency has cleared Braeburn’s Brixadi (buprenorphine) for use in patients with moderate to severe opioid use disorder.
The long-acting injectable features weekly and monthly dosing options for patients who are already being treated with a transmucosal (administered in the mouth) buprenorphine-containing product.
Brixadi's active ingredient is buprenorphine, an old medicine used to treat pain and opioid dependence, according to the National Institutes of Health.
Braeburn’s CEO and president Mike Derkacz called the approval “a significant step forward” in the fight against opioid use disorder.
“Over the last three years the U.S. experienced a significant increase in opioid overdoses in part related to the economic and social upheaval that negatively impacted all of us, especially people with opioid use disorder and people in recovery,” Derkacz said in the company’s statement.
“Additional medication options for opioid use disorder will support healthcare providers in addressing the needs of their patients with opioid use disorder," the CEO added.
Brixadi comes with a boxed warning that highlights risks that can occur if the drug is given administered intravenously. That’s because of its crystalline gel formulation, which could cause severe harm or death if injected through the veins. Instead, the drug must be administered subcutaneously.
The drug should be used alongside counseling and psychosocial support to form a complete treatment plan, the company stressed.
Brixadi will be available in the U.S. starting in September, but only through a restricted distribution program called the Brixadi risk evaluation and mitigation strategy. The program will require healthcare providers to be certified before dispensing the drug.
Most patients will pay $10 or less for the drug, a company spokesperson told Fierce Pharma over email.
Also this week, the FDA approved Indivior’s Opvee, a new option for overdose reversal. That nod came two months after Emergent BioSolution’s won a game-changing approval to sell its Narcan rescue medication over the counter.
Reference:https://www.fiercepharma.com/pharma/braeburns-long-acting-brixadi-cleared-treat-opioid-use-disorder
Read more on
- Anglikang Adenosylcobalamin Capsules Obtain Drug Registration Certificate March 6, 2026
- Two Minoxidil Topical Solution Applications from its Subsidiary Approved March 6, 2026
- Zhejiang Medicine’s ARX305 Initiates Phase II Clinical Trial March 6, 2026
- Indacaterol Mometasone Inhalation Powder Clinical Trial Approved for Asthma Treatment March 6, 2026
- Harsco Pharmaceutical’s innovative drug HSK50042 tablets receive clinical approval for a new indication March 6, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



