-
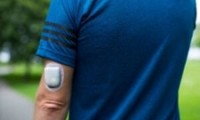
Senseonics gets FDA clearance to pair CGM implant with insulin pumps
- Source: https://www.medtechdive.com/news/senseonics-fda-clearance-integrated-cgm/714804/
- 80
- May 3, 2024
-
Abbott’s latest Heartmate recall tied to 273 injuries, 14 deaths
- Source: drugdu
- 113
- April 17, 2024
-
Intuitive lands FDA clearance for new da Vinci robot
- Source: drugdu
- 131
- March 18, 2024
-
Insulet applauds NICE guidance on hybrid closed loop insulin delivery
- Source: drugdu
- 97
- January 1, 2024
-
Boston Scientific initiates new Farapulse trial, expects FDA approval in 2024
- Source: drugdu
- 186
- December 30, 2023
-
J&J’s Biosense Webster treats first patients in dual-energy AFib ablation trial
- Source: drugdu
- 592
- December 18, 2023
-
Bright Uro Snags $23M To Make Urology Diagnostics Easier for Providers & Patients
- Source: drugdu
- 148
- December 5, 2023
-
J&J links radiofrequency catheter to improved quality of life in atrial fibrillation study
- Source: drugdu
- 100
- November 9, 2023
-
analysts
- Source: drugdu
- 164
- October 11, 2023
-
Should More Doctors Be on TikTok? This One Says Yes
- Source: drugdu
- 220
- October 3, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.