-
Simcere and Connect sign licensing agreement for anti IL-4Rα AD drug
- Source: drugdu
- 188
- November 26, 2023
-
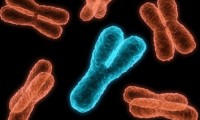
Breakthrough decoding of the Y chromosome revolutionizes digestive disease research
- Source: drugdu
- 189
- November 25, 2023
-
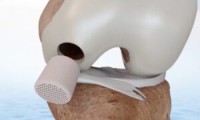
Smith+Nephew to buy CartiHeal and its knee cartilage healing tech
- Source: drugdu
- 136
- November 25, 2023
-
Sunnyside Secures $11.5M To Help People Build Healthier Drinking Habits
- Source: drugdu
- 143
- November 25, 2023
-
Levofloxacin revealed as first effective treatment for multi-drug resistant TB
- Source: drugdu
- 125
- November 25, 2023
-
European Patent Office Declares One of Moderna’s mRNA Patents Invalid
- Source: drugdu
- 105
- November 24, 2023
-
Cosmic radiation and microgravity linked to erectile dysfunction in astronauts
- Source: drugdu
- 95
- November 24, 2023
-
Another medtech reports a cybersecurity incident
- Source: drugdu
- 96
- November 24, 2023
-
EC approves pharmaand’s Rubraca as advanced ovarian cancer maintenance treatment
- Source: drugdu
- 100
- November 24, 2023
-
Novartis drops subpoena request in trade-secret spat with Takeda
- Source: drugdu
- 108
- November 24, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.