-
Ebola vaccines from GSK, Merck elicit yearlong response, study finds
- Source: Fiercepharma
- 796
- October 16, 2017
-
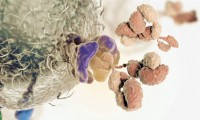
NIH accelerates the development of new cancer immunotherapy strategies for more patients
- Source: nih.gov
- 545
- October 16, 2017
-
China’s Antimicrobial Drug Import and Export Market Report
- Source: Ddu
- 1,400
- October 13, 2017
-
Durable end to the HIV/AIDS pandemic likely will require an HIV vaccine
- Source: Sciencedaily
- 557
- October 13, 2017
-
Cholesterol byproduct hijacks immune cells, lets breast cancer spread
- Source: Medicalxpres
- 716
- October 13, 2017
-
Respicardia gets FDA approval for remede system to treat sleep apnea
- Source: medicaldevice-network
- 517
- October 13, 2017
-
Pfizer reviewing strategic alternatives for consumer healthcare business
- Source: Pfizer
- 523
- October 13, 2017
-
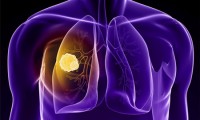
FDA Grants Priority Review To Gilotrif For Uncommon EGFR Mutations In Advanced NSCLC
- Source: careers.biospace
- 541
- October 13, 2017
-
19th Asia Pacific Rheumatology APLAR Congress
- Source: medical-events
- 778
- October 13, 2017
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.