-
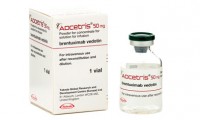
FDA expands approval of Adcetris for first-line treatment of Stage III or IV classical Hodgkin lymphoma in combination with chemotherapy
- Source: finance.yahoo
- 589
- March 22, 2018
-
Bayer receives approval in China for Stivarga® (regorafenib) for the second-line systemic Treatment of liver cancer (for specialized target groups only)
- Source: 1stoncology
- 534
- March 20, 2018
-
Janssen Announces U.S. FDA Breakthrough Therapy Designation for Erdafitinib in the Treatment of Metastatic Urothelial Cancer
- Source: janssen
- 620
- March 19, 2018
-
ROCHE TO BUY FLATIRON HEALTH FOR $1.9 BN TO EXPAND CANCER CARE
- Source: Reuters
- 503
- March 15, 2018
-
IMMUTEP ENTERS INTO CLINICAL TRIAL COLLABORATION AND SUPPLY AGREEMENT WITH MSD
- Source: finance.yahoo
- 630
- March 14, 2018
-
Science 37 and Novartis Sign Strategic Alliance to Advance Decentralized Clinical Trials
- Source: Science 37
- 572
- March 12, 2018
-
Merck and Eisai Ink $5.76B Cancer Deal
- Source: Biospace
- 509
- March 12, 2018
-
Celgene Completes Acquisition of Juno Therapeutics, Inc., Advancing Global Leadership in Cellular Immunotherapy
- Source: ir.celgene
- 589
- March 8, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.