-
U.S. Food and Drug Administration Approves Gilead’s Biktarvy® (Bictegravir, Emtricitabine, Tenofovir Alafenamide) for Treatment of HIV-1 Infection
- Source: gilead
- 906
- February 26, 2018
-
U.S. Food and Drug Administration Approves Gilead’s Biktarvy®
- Source: Finchannel
- 787
- February 9, 2018
-
China Food and Drug Administration Approves Gilead’s Sovaldi® (Sofosbuvir) for Treatment of Chronic Hepatitis C Virus Infection
- Source: Gilead
- 776
- November 27, 2017
-
Over $440 million! Jiahe Biologics’ new dual-antibody drug goes overseas
- Source: drugdu
- 66
- October 11, 2024
-
What is the impact of the House of Representatives’ passage of the draft biosafety law amid the controversy on WuXi
- Source: drugdu
- 79
- October 6, 2024
-
Digital Therapeutics Sector See Billing Codes as Key to Breaking Free of Reimbursement Rut
- Source: drugdu
- 70
- August 21, 2024
-
Ipsen, Genfit Drug That Missed in MASH Wins FDA Approval in a Much Rarer Liver Disease
- Source: drugdu
- 130
- June 13, 2024
-
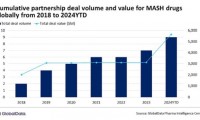
GlobalData
- Source: drugdu
- 131
- April 20, 2024
-
AstraZeneca, Daiichi Sankyo Drug Enhertu Becomes First Tumor-Agnostic ADC Cancer Med
- Source: drugdu
- 86
- April 11, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.