-
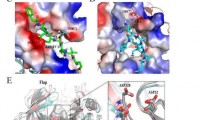
Amgen and TScan Therapeutics partner to identify new Crohn’s disease targets
- Source: drugdu
- 142
- May 16, 2023
-
FDA Approves First-Of-Its Kind Drug to Ease Menopause Symptoms
- Source: drugdu
- 143
- May 16, 2023
-
Abbott expands initiative to increase diversity in clinical trials
- Source: drugdu
- 136
- May 16, 2023
-
FDA greenlights a new type of drug for menopausal hot flashes
- Source: drugdu
- 115
- May 16, 2023
-
Researchers discover novel ‘Shanghai APP’ mutation in late-onset Alzheimer’s disease
- Source: drugdu
- 145
- May 16, 2023
-
Ahead of high-stakes California trial, GSK notches Zantac win in Canada
- Source: drugdu
- 115
- May 16, 2023
-
Study shows AI chatbot provides quality and empathetic answers to patient questions
- Source: drugdu
- 113
- May 16, 2023
-
Steroids linked to long-lasting heart disease risk and worse quality of life
- Source: drugdu
- 107
- May 16, 2023
-
After costly setback, Astellas’ menopause drug crosses FDA finish line
- Source: drugdu
- 106
- May 16, 2023
-
Swiss researchers identify plastic-degrading microbial strains in the Alps and Arctic region
- Source: drugdu
- 205
- May 15, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.