-
Spikevax approved for use in children aged 6 months to 5 years
- Source: drugdu
- 135
- May 11, 2023
-
FDA completes inspection of Nexus’ manufacturing facility in US
- Source: drugdu
- 124
- May 10, 2023
-
Early smoking cessation associated with higher survival rates following a lung cancer diagnosis
- Source: drugdu
- 137
- May 7, 2023
-
Researchers discover a potential cause of Parkinson’s disease
- Source: drugdu
- 127
- May 7, 2023
-
Formus Labs’ Hip Surgery Planning Software Expands with FDA Clearance
- Source: drugdu
- 128
- May 7, 2023
-
WHO announces launch of new pandemic preparedness initiative
- Source: drugdu
- 156
- May 2, 2023
-
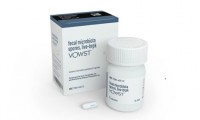
Seres’s Bacteria in a Pill Becomes First FDA-Approved Oral Microbiome Therapy
- Source: drugdu
- 176
- May 2, 2023
-
‘Eat, sleep, console’ reduces hospital stay and need for medication among opioid-exposed infants
- Source: drugdu
- 134
- May 2, 2023
-
Long siesta-takers have higher BMIs and more likely to have metabolic syndrome
- Source: drugdu
- 140
- May 1, 2023
-
Exploring Doggybone ™ DNA Technology
- Source: drugdu
- 118
- April 30, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.