-
New Sample Preparation Solutions Simplify and Automate Respiratory Diagnostic Testing
- Source: drugdu
- 108
- December 15, 2023
-
Glycan analysis holds promise for early diagnosis of various cancers
- Source: drugdu
- 98
- December 15, 2023
-
MAPS Seeks Approval of First Psychedelic-Assisted Therapy for PTSD
- Source: drugdu
- 96
- December 15, 2023
-
Sanofi Abandons Deal for Rare Disease Drug Amid FTC’s Monopoly Concerns
- Source: drugdu
- 100
- December 15, 2023
-
Sino Biopharm’ S&P ESG Score Gains Another Boost, Stays in Top 9% Globally
- Source: drugdu
- 95
- December 15, 2023
-
Mayor of Bogota, Colombia, Claudia Lopez and Ambassador of Colombia, Sergio Cabrera and his entourage visited SINOVAC.
- Source: drugdu
- 91
- December 15, 2023
-
126 new drugs were included, with an average price reduction of 61.7% during negotiations…
- Source: drugdu
- 120
- December 15, 2023
-
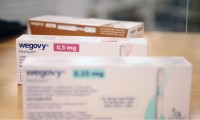
BMJ probe questions oversight of UK pharmacy materials on Wegovy amid illegal ad accusations
- Source: drugdu
- 194
- December 15, 2023
-
After swell of biopharma outrage, Supreme Court takes up high-profile mifepristone case
- Source: drugdu
- 119
- December 15, 2023
-
【EXPERT Q&A】What is a pharmaceutical intermediate?
- Source: drugdu
- 239
- December 15, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.