-
Thermo Fisher to cut the lights at California plastics plant, lay off 74
- Source: drugdu
- 92
- January 6, 2024
-
Inbound HCP communication channels double digital engagement, Veeva finds
- Source: drugdu
- 89
- January 6, 2024
-
Bluebird bio’s Lyfgenia launch progresses with 2nd major outcomes-based coverage deal
- Source: drugdu
- 82
- January 6, 2024
-
Novo Nordisk, Eli Lilly’s weight-loss drugs under FDA scrutiny for suicidal thoughts, hair loss
- Source: drugdu
- 88
- January 6, 2024
-
CVS Caremark to kick AbbVie’s Humira off some formularies in favor of cheaper biosimilars
- Source: drugdu
- 135
- January 6, 2024
-
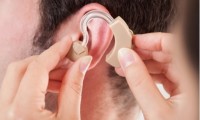
Wearing hearing aids could extend people’s lifespan
- Source: drugdu
- 165
- January 5, 2024
-
AI Tool Rapidly Analyzes Gene Activities in Medical Images to Highlight Hidden Cancers
- Source: drugdu
- 90
- January 5, 2024
-
FDA Fast Tracks Candel Therapeutics’ Novel Treatment for Pancreatic Ductal Adenocarcinoma
- Source: drugdu
- 170
- January 5, 2024
-
BIO-THERA Signs License and Commercialization Agreement with Macter for BAT1706
- Source: drugdu
- 80
- January 5, 2024
-
Henlius to Release Clinical Data at 2024 ASCO GI
- Source: drugdu
- 130
- January 5, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.