-
BeiGene’s Brukinsa granted FDA accelerated approval for advanced follicular lymphoma
- Source: drugdu
- 75
- March 12, 2024
-
Achieving first full year of profitability, and ushering in a new phase of high-quality development
- Source: drugdu
- 126
- March 10, 2024
-
FDA Approves Opdivo Plus Chemotherapy for Frontline Treatment of Unresectable, Metastatic Urothelial Carcinoma
- Source: drugdu
- 126
- March 9, 2024
-
CPhI Japan 2024
- Source: drugdu
- 174
- March 8, 2024
-
Gilead Sciences Signs Yet Another R&D Alliance for Multi-Target Cancer Therapies
- Source: drugdu
- 105
- March 8, 2024
-
FDA Approves Sandoz Biosimilars for Two Blockbuster Amgen Bone Drugs
- Source: drugdu
- 134
- March 7, 2024
-
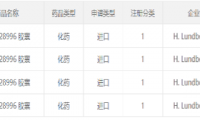
Lundbeck Pharmaceuticals’ “Anti-Parkinson’s Category 1 Chemical Drug” Submits Clinical Application in China
- Source: drugdu
- 158
- March 7, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.