-
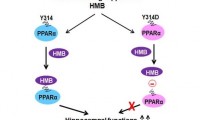
Bodybuilding supplement may help stave off progression of Alzheimer’s
- Source: drugdu
- 115
- July 22, 2023
-
Eisai, Biogen’s Leqembi May Face Rollout Hurdles Now, but Experts Still Like the Alzheimer’s Drug
- Source: drugdu
- 101
- July 15, 2023
-
New program offers hiking adventures for people with younger-onset Alzheimer’s
- Source: drugdu
- 115
- July 10, 2023
-
With full Alzheimer’s approval in hand, Eisai and Biogen kick off Leqembi’s launch in earnest
- Source: drugdu
- 110
- July 8, 2023
-
Missed trial protocols, fabricated emails and failed endpoint mar BioXCel’s Alzheimer’s agitation readout
- Source: drugdu
- 105
- July 2, 2023
-
Eisai, Biogen’s Alzheimer’s disease drug Leqembi passes muster at FDA adcomm
- Source: drugdu
- 111
- June 13, 2023
-
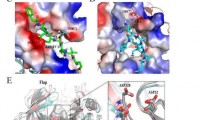
Researchers discover novel ‘Shanghai APP’ mutation in late-onset Alzheimer’s disease
- Source: drugdu
- 147
- May 16, 2023
-
Otsuka, Lundbeck’s Rexulti nabs new use in Alzheimer’s disease agitation
- Source: drugdu
- 111
- May 13, 2023
-
Spherix
- Source: drugdu
- 132
- April 14, 2023
-
FDA Grants Breakthrough Therapy Designation to Pfizer’s Investigational Alzheimer’s Disease Treatment
- Source: drugdu
- 250
- March 22, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.