-
Profound Medical, Siemens Healthineers collab on ultrasound ablation
- Source: drugdu
- 88
- March 4, 2024
-
Tandem cycling boosts well-being for Parkinson’s patients and care partners
- Source: drugdu
- 93
- March 4, 2024
-
Comprehensive protein analysis reveals potential targets for treating dialysis-related amyloidosis
- Source: drugdu
- 131
- March 4, 2024
-
New Hantavirus Rapid Test Paves Way for Early Outbreak Control
- Source: drugdu
- 101
- March 2, 2024
-
Nuvalent Granted FDA Breakthrough Therapy Designation for Non–Small Cell Lung Cancer Drug
- Source: drugdu
- 87
- March 2, 2024
-
‘Top-down’ treatment strategy dramatically improves outcomes for Crohn’s patients
- Source: drugdu
- 126
- March 2, 2024
-
【EXPERT Q&A】How to apply for Biologics License Application (BLA)?
- Source: drugdu
- 144
- February 29, 2024
-
Simple Measurement Predicts Risk of Rapid Progression of Chronic Kidney Disease
- Source: drugdu
- 95
- February 29, 2024
-
FDA Approves Imbruvica Label Expansion to Include Oral Suspension Formulation
- Source: drugdu
- 129
- February 29, 2024
-
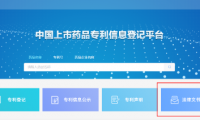
China’s marketed drug patent information registration platform has a new “legal document submission module”
- Source: drugdu
- 86
- February 29, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.