-
Viela Bio Spins Out of MedImmune; Raises Up to $250 Million in Series A Financing, 6 Dimensions Capital Leads Investment
- Source: finance.yahoo
- 475
- March 1, 2018
-
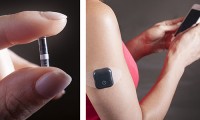
Implanted continuous glucose sensor proven safe
- Source: globalbiotechinsights
- 714
- February 28, 2018
-
Cedars-Sinai, Emulate partner to give precision medicine a boost with organs-on-chips tech
- Source: Healthcare IT News
- 548
- February 27, 2018
-
AI Biotech XtalPi Closes Series B Round Funding from Sequoia China, Google, and Tencent
- Source: finance.yahoo
- 523
- February 26, 2018
-
Merck Drops $394 Million to Acquire Virus-Based Cancer Company Viralytics
- Source: Biospace;
- 726
- February 26, 2018
-
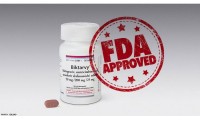
U.S. Food and Drug Administration Approves Gilead’s Biktarvy® (Bictegravir, Emtricitabine, Tenofovir Alafenamide) for Treatment of HIV-1 Infection
- Source: gilead
- 906
- February 26, 2018
-
Choices for Your Ideal Medical Hospital Beds
- Source: Ddu
- 976
- February 26, 2018
-
What’s Driving the Global Surgical Robotics Market
- Source: marketresearch.com
- 1,224
- February 26, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.