-
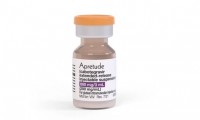
MSF, trying to secure supplies of ViiV’s Apretude, calls out new clauses in purchasing contract
- Source: drugdu
- 135
- August 19, 2023
-
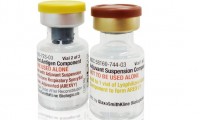
GSK stays a step ahead in RSV vaccine battle as Arexvy becomes available at US pharmacies
- Source: drugdu
- 173
- August 19, 2023
-
Legend exec
- Source: drugdu
- 120
- August 18, 2023
-
Multimillion-dollar NIH grant awarded to develop advanced treatment for diabetic foot ulcers
- Source: drugdu
- 125
- August 17, 2023
-
Pfizer Bispecific Drug Wins FDA Accelerated Approval for Multiple Myeloma
- Source: drugdu
- 216
- August 17, 2023
-
FDA Approves J&J’s Akeega for BRCA-Positive Prostate Cancer
- Source: drugdu
- 187
- August 16, 2023
-
Janssen’s Talvey granted FDA accelerated approval for difficult-to-treat blood cancer
- Source: drugdu
- 170
- August 15, 2023
-
Supreme Court blocks Purdue Pharma’s bankruptcy settlement, threatening immunity to Sackler family
- Source: drugdu
- 110
- August 15, 2023
-
Boston Scientific receives FDA approval for cryoablation system
- Source: drugdu
- 150
- August 12, 2023
-
J&J signs Stelara biosimilar settlement deal with Formycon and Fresenius
- Source: drugdu
- 194
- August 10, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.