-
Phesi’s AI-driven Trial Accelerator platform contains over 100 million patients
- Source: drugdu
- 97
- March 16, 2024
-
First UK patients receive experimental mRNA therapy for cancer in global trial
- Source: drugdu
- 81
- March 16, 2024
-
Hengrui Medicine’s new “PARP inhibitor” HRS-1167 combination therapy for advanced solid tumors received clinical approval
- Source: drugdu
- 157
- March 15, 2024
-
Trial Shows Lilly’s Lebrikizumab Produces Improvements in Atopic Dermatitis Severity, Particularly in Individuals with Skin of Color
- Source: drugdu
- 105
- March 14, 2024
-
Landmark Clinical Approval for YOLT-201 Obtained by the NMPA
- Source: drugdu
- 87
- March 10, 2024
-
AACR 2024 | Ascentage Pharma to Present Three Preclinical Studies at 2024 American Association of Cancer Research Annual Meeting
- Source: drugdu
- 94
- March 10, 2024
-
ViiV Healthcare Reveals Positive Results from Phase I Trial for a Potential Longer-Acting HIV Treatment
- Source: drugdu
- 76
- March 8, 2024
-
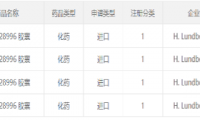
Lundbeck Pharmaceuticals’ “Anti-Parkinson’s Category 1 Chemical Drug” Submits Clinical Application in China
- Source: drugdu
- 153
- March 7, 2024
-
AstraZeneca’s “CD19/CD3 dual antibody” receives clinical approval in China
- Source: drugdu
- 92
- March 2, 2024
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.