-
Boehringer Ingelheim advances weight loss drug to Phase III trials
- Source: drugdu
- 102
- August 22, 2023
-
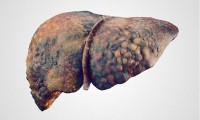
Meta-analysis reports global prevalence of MAFLD of 38.8% in adults
- Source: drugdu
- 185
- August 21, 2023
-
Novo Nordisk spends $1bn to acquire weight loss drug developer Inversago.
- Source: drugdu
- 228
- August 21, 2023
-
Wegovy could prevent up to 1.5 million heart attacks, strokes over 10 years, study says
- Source: drugdu
- 199
- August 18, 2023
-
Novo Nordisk spends $1bn to acquire weight loss drug developer Inversago.
- Source: drugdu
- 117
- August 12, 2023
-
Wegovy shown to reduce risk of heart attack, stroke in major cardiovascular trial
- Source: drugdu
- 128
- August 11, 2023
-
Novo’s Wegovy Protects Heart Health in Overweight, Obese Adults
- Source: drugdu
- 114
- August 10, 2023
-
Artificial sweeteners linked to increased body fat, study finds
- Source: drugdu
- 263
- August 7, 2023
-
Novo Nordisk, Eli Lilly face injury lawsuit from user of popular GLP-1 medicines
- Source: drugdu
- 117
- August 7, 2023
-
UK Probes Weight Loss, Diabetes Drugs for Suicidal Ideation and Self-Harm Risk
- Source: drugdu
- 119
- July 28, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.