-
After launch limitations, Johnson & Johnson exec touts manufacturing progress on CAR-T drug Carvykti
- Source: drugdu
- 108
- October 20, 2023
-
Ultimovacs Rebounds on New Universal Cancer Vaccine Data for Mesothelioma
- Source: drugdu
- 103
- October 20, 2023
-
CHMP recommends European authorisation for seven new drugs
- Source: drugdu
- 108
- October 19, 2023
-
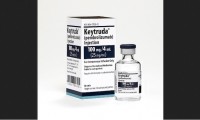
Merck’s Keytruda receives approval greenlight from EU CHMP
- Source: drugdu
- 170
- October 18, 2023
-
Novo Nordisk Strikes Deal for Late-Stage Hypertension & Kidney Disease Drug
- Source: drugdu
- 218
- October 18, 2023
-
High-flying Novo Nordisk again dials up its 2023 sales and profit expectations
- Source: drugdu
- 191
- October 17, 2023
-
Interest in RNA Editing Accelerates as Therapies Approach the Clinic
- Source: drugdu
- 133
- October 17, 2023
-
Akero’s Three-Step Plan to Win Back Investors
- Source: drugdu
- 115
- October 16, 2023
-
Biogen’s Aduhelm headache returns as appeals court revives part of investor lawsuit
- Source: drugdu
- 132
- October 14, 2023
-
Lilly’s Mirikizumab Shows Long-Term Remission in Phase III Crohn’s Trial
- Source: drugdu
- 107
- October 14, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.