-
Pfizer’s Paxlovid approved by FDA and Gilead’s Veklury recommended by CHMP
- Source: drugdu
- 105
- May 30, 2023
-
Pfizer oral weight loss drug may be as effective as Ozempic injection by Novo Nordisk, study says
- Source: drugdu
- 148
- May 24, 2023
-
CureVac seeks to accelerate vaccine infringement case against Pfizer and BioNTech
- Source: drugdu
- 114
- May 23, 2023
-
Pfizer puts kibosh on 4 antibiotics in India after contractor’s manufacturing issues
- Source: drugdu
- 115
- May 20, 2023
-
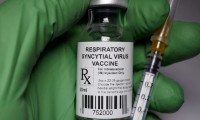
FDA advisors recommend Pfizer’s RSV vaccine for infants but raise safety concerns
- Source: drugdu
- 113
- May 20, 2023
-
Pfizer to raise $31 billion in debt offering to fund Seagen acquisition, SEC filing shows
- Source: drugdu
- 127
- May 19, 2023
-
Pfizer CEO says Medicare will likely face legal action over drug price negotiations
- Source: drugdu
- 115
- May 13, 2023
-
Pfizer Acquires COVID-Flu Test Developer Lucira Health for USD 36.4 Million
- Source: drugdu
- 120
- May 5, 2023
-
Pfizer CEO’s texts with European Commission chief trigger new NYT lawsuit
- Source: drugdu
- 139
- May 2, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.