-
Moderna says it expects up to $15 billion in sales of Covid, RSV, flu vaccines in 2027
- Source: drugdu
- 120
- May 2, 2023
-
Medicare will cover Leqembi for all patients if FDA approves drug, CMS chief says
- Source: drugdu
- 116
- May 1, 2023
-
Moderna says it expects up to $15 billion in sales of Covid, RSV, flu vaccines in 2027
- Source: drugdu
- 136
- April 30, 2023
-
Hansa Biopharma enters into collaboration with Genethon
- Source: drugdu
- 124
- April 30, 2023
-
BrainStorm files ALS drug application over FDA protest
- Source: drugdu
- 169
- April 28, 2023
-
Biogen’s new strategy brings more pipeline cuts, but leaves deal options open
- Source: drugdu
- 177
- April 27, 2023
-
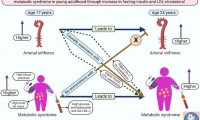
Arterial stiffness may be a novel risk factor for childhood and adolescent metabolic disease
- Source: drugdu
- 102
- April 27, 2023
-
Study shows no significant cognitive benefit of adhering to Mediterranean diets regardless of calorie intake
- Source: drugdu
- 126
- April 27, 2023
-
After delay, Bluebird submits sickle cell gene therapy for FDA approval
- Source: drugdu
- 163
- April 26, 2023
-
Apellis’ immune system drug hits a setback in ALS
- Source: drugdu
- 162
- April 25, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.