-
Spikevax approved for use in children aged 6 months to 5 years
- Source: drugdu
- 130
- May 11, 2023
-
Life Bio, Forge to develop gene therapies for aging-linked diseases
- Source: drugdu
- 117
- May 11, 2023
-
Researchers evaluate the dual-therapeutic effect of gene therapy in mouse model of osteosarcoma
- Source: drugdu
- 142
- May 9, 2023
-
The safety and efficacy of FINLAY-FR-2/1A, a new protein-based COVID-19 vaccine
- Source: drugdu
- 124
- May 9, 2023
-
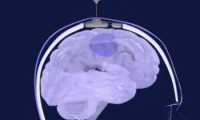
Researchers use skull-implantable ultrasound to help deliver chemotherapy to the brain
- Source: drugdu
- 112
- May 7, 2023
-
Algorithm generates 128-fold increase in antibodies
- Source: drugdu
- 237
- May 5, 2023
-
Bristol Myers Squibb and Tubulis partner in deal worth over $1bn
- Source: drugdu
- 140
- May 5, 2023
-
ChatGPT outshines physicians in quality and empathy for online patient queries
- Source: drugdu
- 139
- May 5, 2023
-
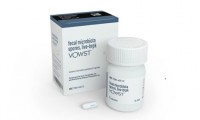
Seres’s Bacteria in a Pill Becomes First FDA-Approved Oral Microbiome Therapy
- Source: drugdu
- 169
- May 2, 2023
-
‘Eat, sleep, console’ reduces hospital stay and need for medication among opioid-exposed infants
- Source: drugdu
- 129
- May 2, 2023
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.