Remigipam intermediates series
August 1, 2025
Source: drugdu
 203
203
Migraine is a common chronic neurovascular disease, ranked by the World Health Organization as the third most common and second most disabling neurological disease worldwide. According to statistics, approximately 1.3 billion migraine sufferers worldwide, with approximately 47 million cases in the United States. China has the highest number of migraine sufferers, with over 132 million patients. IQVIA (IQVIA, a leading global healthcare information and strategic consulting company) predicts that the global migraine market is expected to exceed US$13.2 billion in 2026, and sales of calcitonin gene-related peptide (CGRP)-targeted drugs for migraine treatment are expected to exceed US$6.5 billion in 2027.
In this field, Remigipam is a milestone. It is the world's only CGRP receptor antagonist that uses patented orally disintegrating tablet technology. It is also the world's first drug that can be used for both acute treatment and preventive treatment of migraine attacks.
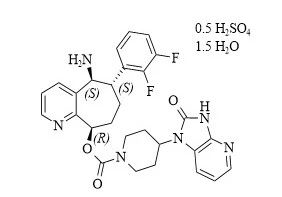
In February 2020, Remdesivir orally disintegrating tablets were approved in the United States and were marketed by Biohaven Pharmaceutical Holding Co Ltd; in May 2022, Pfizer acquired Biohaven for a total of approximately US$11.6 billion and incorporated the drug into its portfolio; on January 23, 2024, Remdesivir was approved by the China National Medical Products Administration and officially entered the Chinese market.
Judging from sales performance, the market recognition of Remigipam continues to increase: in 2020, Biohaven's global sales of Remigipam were US$63 million, which increased to US$462 million in 2021; after being acquired by Pfizer, global sales were US$213 million in 2022, which rose sharply to US$928 million in 2023, and further increased to US$1.263 billion in 2024.
Amidst the rapidly growing market for Remigipam, high-quality pharmaceutical intermediates are crucial for ensuring large-scale production and quality control for downstream customers. Leveraging its proprietary enzyme catalysis technology, Shangke Bio has successfully developed a series of core Remigipam intermediates, including CAS No. 1190363-46-2 (enzymatic), CAS No. 1373116-07-4 (enzymatic), CAS No. 781649-84-1, CAS No. 1190363-44-0 (enzymatic), CAS No. 1374024-48-2, CAS No. 1190363-45-1 (enzymatic), and CAS No. 1373116-06-3, which can be used in the synthesis of Remigipam.
Source:https://news.yaozh.com/archive/45848.html
By editorRead more on
- Rovaxitinib approved for marketing, filling the demand for myelofibrosis treatment March 2, 2026
- Warrant Pharmaceuticals’ active pharmaceutical ingredient receives Brazil’s first official GMP certification March 2, 2026
- Merck’s New Story March 2, 2026
- Rongchang Biotechnology has turned a profit! March 2, 2026
- Jiuyuan Gene’s “Simeglucopyranoside” for weight loss (Jikeqin®) has been submitted for market approval March 2, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



