LifePlus announces glucose monitoring wearable
May 18, 2018
Source: Ddu
 1,360
1,360
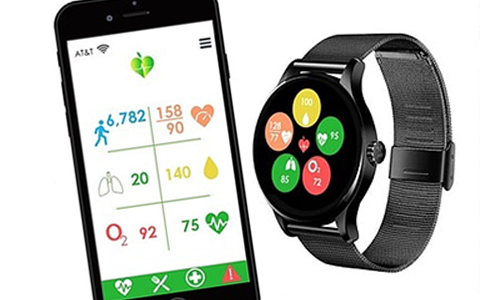
Now you can monitor your blood glucose levels just by looking at your wrist if LifePlus has its way. The startup has announced what it has hailed as the first noninvasive continuous blood glucose monitoring multi-sensor wearable.
Named LifeLeaf, the device has the potential to revolutionize the mushrooming medical wearables segment.
The company announced that the innovative product, whose technological details are not known yet, is undergoing clinical trials in five cities across the world.
The patent-pending invention is based on open standards software and cloud-based analytics. The system gives users real-time notifications and cloud-based AI solutions.
John Trobough, executive chairman of the board, said that the startup, which had been in stealth mode, is excited to announce the introduction of LifeLeaf. He stressed the importance of early detection and management of diabetes and other chronic diseases. He termed LifeLeaf as a unique product and software technology available. “It is truly unique and we are excited to make this multi-sensor capability available to companies and developers globally,” he said.
The multi-sensor smartwatch contains embedded algorithms which extract key parameters, which are then sent to the user’s smartphone interface. Based on the glucose levels, users can decide if they want to alert their doctor.
The device can non-invasively monitor heart rates, chronic health conditions including diabetes, cardiac arrhythmia, congestive heart failure, COPD, sleep apnea, hypertension, respiration rate, blood pressure, and oxygen saturation.
LifeLeaf and LifePlus don’t have clearance from the FDA. The company claims to have the first noninvasive continuous blood glucose monitoring multi-sensor wearable. However, players like Prediktor Medical also have similar wearables currently undergoing development and testing.
By editorRead more on
- 【EXPERT Q&A】Is it mandatory to conduct clinical trials for drug registration in India? August 9, 2023
- 4 Wearable ECGs EKGs Monitor and Their Features September 5, 2018
- Wearable Medical Device to Promote Maternal and Fetal Health in Late Pregnancy September 4, 2018
- Merck’s New Digital Diabetes Coaching to Educate and Manage Patients August 31, 2018
- UK Advertising Regulator Bans Natural Cycles’ Facebook Ad August 31, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



