Medical registration for India —— What to do after the first official submission?
August 24, 2021
Source: drugdu
 1,427
1,427

People who understand the process of medical registration for India would know that Indian registration is divided into two steps, which are Marketing Approval and Product Registration.You can find the details of the registration profile and flowchart from different countries we have prepared for you on Ddu (Drugdu.com).
Ddu has listed the key points about what we need to do after the first official submission of Marketing Approval documents.
1. Wait for the official feedback, and follow the feedback to make the corresponding data revisions and supplements;
2. Get prepared for the plan of clinical Phase; You may have doubts while reading this point, why do we need to prepare a clinical plan during the first stage of registration, which is the Marketing Approval stage?
First, the clinical Phase 3 study is a major premise for vaccine products to enter the Indian market;
Secondly, the review of sample and clinical Phase 3 data is also an indispensable part of documents to the Indian Marketing Approval stage, so we have to complete this part of the trial before the market access permit is issued.
3. The plan of clinical phase 3 and sample test application will be submitted to the relevant official, wait for approval;
4. After the approval of Phase 3 and the sample application, the sample will be sent to the Indian department of CRI KASAULI for testing;
5. When the sample test was passed, a clinical phase 3 trial will be conducted in India;
6. We will obtain Form 45 after all the information within the Marketing Approval stage has been approved;
7. Subsequently, we will be ready to submit the relevant documents regarding the Product Registration stage and wait for the reviewing process, after which we can get Form 41.
8. As the final step, the product packaging materials details will be submitted and wait for the approval to pass, after which we can obtain Form 10, and then it’s time to officially export the products.
P.S In regard to the forms which are mentioned within the point 6 to point 8, please click here for more details. ( Link to Rules of Indian drug registration The essential Forms for occupying the Indian pharmaceutical market )
Here is the point: When is it appropriate to prepare for the Indian Phase 3 plan? The answer is -- the sooner the better. As for the reason, Ddu shows the flow chart below to assist you to understand.
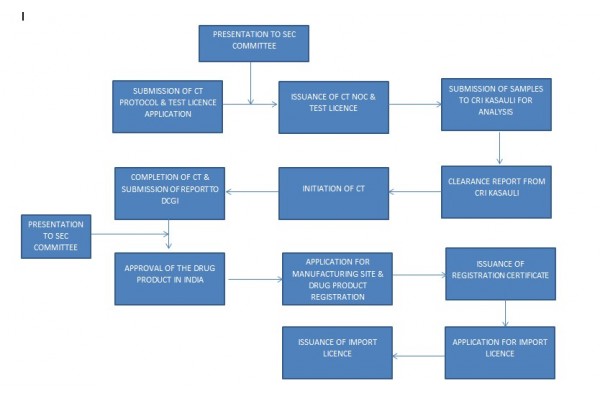 According to the figure, we can understand that the plan of the Indian Phase 3 needs to be submitted to the office along with the sample application, and the sample will be sent to the Indian CRI KASAULI for testing after the clinical phase 3 and sample application are approved. The preparation of the clinical plan usually takes longer, so after we formally confirm the cooperation, we can initially formulate the plan that needs to be carried out in India according to the domestic clinical plan, which can greatly shorten the time-consuming of the entire registration.
According to the figure, we can understand that the plan of the Indian Phase 3 needs to be submitted to the office along with the sample application, and the sample will be sent to the Indian CRI KASAULI for testing after the clinical phase 3 and sample application are approved. The preparation of the clinical plan usually takes longer, so after we formally confirm the cooperation, we can initially formulate the plan that needs to be carried out in India according to the domestic clinical plan, which can greatly shorten the time-consuming of the entire registration.
Read more on
- API Is Not Just a Cost – It’s Your Ticket to Global Markets March 3, 2026
- Driven by drugs for lowering blood sugar and losing weight, the export value of Chinese Western medicine preparations has reached a record high February 2, 2026
- Chengdu Pioneer and Kangzhe Pharmaceutical have reached a cooperation agreement to leverage the DEL and HAILO platforms to empower the development of multi-target innovative drugs February 2, 2026
- J&J’s subcutaneous monoclonal antibody combination therapy receives further FDA approval; Autoimmune CAR-T therapy granted FDA Breakthrough Therapy Designation February 2, 2026
- Why is the development of AI-based antiviral drugs a niche field yet a necessity? February 1, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



