Ascentage Pharmaceuticals is out with its powerful weapon
July 15, 2025
Source: drugdu
 193
193
On July 10, according to the official website of NMPA, Ascentage Pharma's highly anticipated pipeline product, lisatoclase tablets (APG-2575), has been conditionally approved for marketing for adult chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) patients who have previously received at least one systemic treatment including a Bruton's tyrosine kinase (BTK) inhibitor.
You should know that before this, there was only one Bcl-2 inhibitor in the world - venetoclax. Since then, the research and development boom of Bcl-2 drugs can be said to have lasted for ten years. Now, it has finally borne fruit in the biotech company Ascentage Pharmaceuticals.
A major target for crossing the cycle
Bcl-2 is the founding member of the Bcl-2 regulatory protein family, the most notable feature of which is its regulatory role in mitochondrial apoptosis. The Bcl-2 family of proteins consists of members that promote or inhibit apoptosis, and controls apoptosis by controlling mitochondrial outer membrane permeability (MOMP), which is a key step in the intrinsic pathway of apoptosis. It can be said that the interaction between members of the BCL-2 protein subgroup determines whether the cell undergoes apoptosis or survives. As of 2008, a total of 25 genes have been identified in the Bcl-2 family.
In this family, Bcl-2 protein belongs to the type that inhibits cell apoptosis. Although the apoptosis process is very finely regulated in normal processes, in cancer, especially in the case of hematological tumors, this process will fall into disorder or even be destroyed. According to the literature, chromosomal translocation will lead to the dysregulated overexpression of Bcl-2 in human follicular lymphoma (FL). In addition, high levels of BCL-2 have also been detected in several other hematological malignancies, including chronic lymphocytic leukemia (CLL), diffuse large B-cell lymphoma (DLBCL) and mantle cell lymphoma; according to the literature "Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2", overexpression of BCL-2 greatly accelerates the development of c-MYC-driven lymphoma in mice.
After discovering the overexpression of the target, the next step is to study related drugs. Before APG-2575, the only drug on the market was AbbVie's venetoclax, which can be traced back to ABT-263 (navitoclax), which targets Bcl-2 and Bcl-XL, but the drug showed dose-limiting severe thrombocytopenia in Phase I clinical trials. People found that BCL-XL inhibition led to reduced platelet survival, so they modified the drug structure and constructed a new type of molecule that has a specific effect only on Bcl-2. In this way, venetoclax was born.
According to the Phase III clinical trial of venetoclax in chronic lymphocytic leukemia (CLL), after a median follow-up period of 38.8 months, the interim analysis of mPFS showed that the combination of venetoclax-obinutuzumab-ibrutinib was superior to chemoimmunotherapy, with an HR of 0.32; P<0.001, and the difference in efficacy was significant.
The drug was successfully approved by the FDA in 2016 for the treatment of chronic lymphocytic leukemia (CLL) patients with 17p deletion mutations. In 2024, the drug's sales reached US$2.583 billion, marking the beginning of a new era.
Knives Out
After venetoclax was launched, there was no successor for nearly a decade, and only now has the second drug, lisatoclax tablets (APG-2575), been approved for marketing. APG-2575 has obtained five orphan drug designations from the FDA, namely Waldenstrom's macroglobulinemia, chronic lymphocytic leukemia, multiple myeloma, acute myeloid leukemia, and follicular lymphoma. They are all very classic bone marrow leukemias, and are also indications that are easier to produce results compared with chemotherapy.
The first thing to mention is the first human clinical trial. The first clinical trial got off to a good start: although there were many adverse reactions, no patient stopped taking the drug due to adverse reactions. In addition, in terms of efficacy, clinical pharmacokinetic and pharmacodynamic results showed that lisaftoclax had limited plasma residence time and systemic exposure, and could quickly eliminate malignant cells. Among the 22 R/R CLL/SLL patients who could be evaluated for efficacy, the median treatment cycle was 15 (range: 6-43) cycles, of which 14 achieved partial remission, with an objective remission rate of 63.6%. This also laid a solid foundation for the subsequent phase II clinical trials.
After that, it was the Phase Ib clinical trial, which was also for relapsed/refractory chronic lymphocytic leukemia or small lymphocytic lymphoma (R/R CLL/SLL). 45 patients were enrolled in the Phase II clinical trial, and no DLT and MTD were observed. The median (range) treatment was 7 (1-17) cycles, and the median (range) onset time was 1 (1-13) cycle. Among the 41 evaluable CLL patients, 1 achieved complete remission (CR), 27 CLL/SLL patients achieved partial remission (PR), and the objective response rate (ORR) was 68.29%. The most important issue for this clinical trial is safety: 13 patients experienced serious adverse reactions, and 14 patients stopped treatment due to adverse reactions. However, the breakthrough of this clinical trial is to determine the optimal dose for the Phase II clinical trial: 600 mg.
Later, the data from two clinical Phase Ib studies were updated. As of April 27, 2023, a total of 47 patients received daily escalating doses of APG-2575, ranging from 100 to 800 mg per day. At baseline, 66.0% and 44.7% of patients had received ≥2 or 3 lines of systemic treatment, 23.4% of patients had received ≥1 BTK inhibitor, and 55.3% of patients had received CD20 antibody treatment. In terms of final efficacy: the overall response rate (ORR) was 73.3% (33/45), and the complete remission (CR)/complete remission (CRi) rate was 24.4% (11/45). And this time a very important conclusion was reached: it seems that the CR rate is dose-dependent.
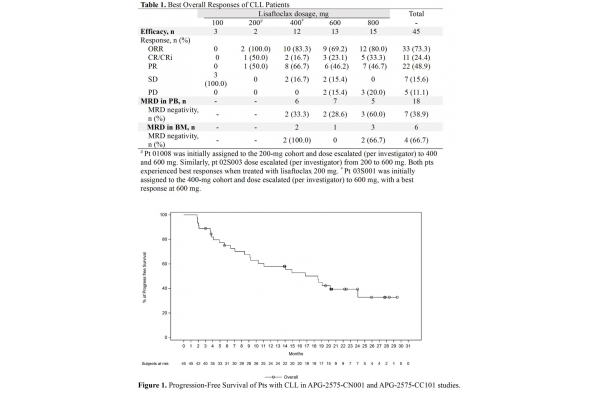
Later, at the 2024 ASH Annual Meeting, the better highlight of the drug was its combination with acalabrutinib: among 87 patients, the ORR of lisaftoclax combined with acalabrutinib treatment reached 96.6%.
In addition, at the 2024 ASH Annual Meeting, APG-2575 also released preliminary clinical results for the treatment of MDS, with a total of 49 patients enrolled: 8 with R/R MDS and 41 with TN MDS. In terms of ORR, the ORR of the 8 R/R MDS patients reached 75%, and the ORR of the 41 TN MDS patients reached an ORR of 77.5%.
This is probably all the valuable clinical progress that we can see at present. We can all wait for the good news from Ascentage for the Phase III clinical data.
Ascentage Pharmaceuticals' reserve force
As a biotech company with a relatively high market value, Ascentage Pharmaceuticals is certainly supported by more than just its two major products, olibactinib and APG-2575, and there will definitely be new forces behind it.
The first one worth mentioning is APG-115, which is an MDM2-p53 inhibitor used to treat advanced adenoid cystic carcinoma (ACC). Phase II clinical data have just been released: among the 17 patients in the monotherapy group whose efficacy could be evaluated, the ORR of 12 ACC patients was 16.7%, and the DCR was 100%; in the combination group with toripalimab, the ORR of 6 BTC patients was 16.7%, and the DCR was 100%; the ORR of 6 LPS patients was 16.7%, and the DCR was 66.7%.
Then the more interesting pipeline is APG-1252, because its target is Bcl-2/Bcl-xL, these two targets are the pipeline that AbbVie failed in the previous article, because it can cause severe thrombocytopenia. Now Ascent Pharmaceuticals not only picked it up, but also used it to treat solid tumors, such as non-small cell lung cancer and small cell lung cancer, and combined with osimertinib. Its clinical phase I ORR is 25%, and the efficacy needs to be observed later.
In addition, APG-2449 is a pipeline that can be valued. It is a FAK/ALK/ROS1 inhibitor with the main indications of non-small cell lung cancer and ovarian cancer. According to the APG-2449 data, among 22 NSCLC patients who were resistant to the second-generation ALK inhibitors and had no targetable bypass gene mutations (such as KRAS G12C, BRAF V600E), 9 cases (9/22, 40.9%) achieved PR. For later-line treatment, the data can be said to be very good. In the second-phase recommended dose group, 9 out of 12 patients achieved intracranial PR.
This is roughly what Ascentage Pharmaceuticals’ new force is like.
Conclusion: The launch of Ascentage Pharma’s Bcl-2 inhibitor may not only represent a breakthrough in a single pipeline, but also represent that China’s innovative drugs are entering a new era and will eventually shine in the global innovative drug market.
Source:https://news.yaozh.com/archive/45766.html
By editorRead more on
- Rovaxitinib approved for marketing, filling the demand for myelofibrosis treatment March 2, 2026
- Warrant Pharmaceuticals’ active pharmaceutical ingredient receives Brazil’s first official GMP certification March 2, 2026
- Merck’s New Story March 2, 2026
- Rongchang Biotechnology has turned a profit! March 2, 2026
- Jiuyuan Gene’s “Simeglucopyranoside” for weight loss (Jikeqin®) has been submitted for market approval March 2, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



