$10 billion! Merck bets on respiratory drugs
July 15, 2025
Source: drugdu
 270
270
On July 9, Merck announced that it had reached a final agreement with Verona Pharma (VRNA), a biopharmaceutical company focusing on respiratory diseases. According to the agreement, Merck will acquire Verona's American Depositary Shares (ADS) through a subsidiary at a price of US$107 per share, with a total transaction value of approximately US$10 billion.
Through this acquisition, Merck will acquire Ohtuvayre, a first-in-class new COPD drug, expanding its R&D pipeline and product portfolio for the treatment of cardiopulmonary diseases, which is expected to drive growth over the next decade.

This is not the first time Merck has made a big investment.
According to public data, the patent for PD-1 inhibitor Keytruda, which accounts for nearly half of Merck's revenue, is about to expire, and the US government is expected to negotiate for Keytruda under the IRA in 2026. Another best-selling drug of Merck, HPV vaccine Gardasil, is facing severe challenges from the fluctuation of demand in the Chinese vaccine market, ending its growth trend ahead of schedule.
As its two main products have faced uncertainty one after another, Merck has accelerated its investment in recent years to expand its R&D pipeline, including the $11.5 billion acquisition of Acceleron, the $10.8 billion acquisition of Prometheus Biosciences, and this $10 billion acquisition of Verona.
According to information, Verona Pharma was founded in 2005 and is a London-based biotechnology company focused on the development and commercialization of drugs for chronic respiratory diseases. Ohtuvayre is the company's first commercialized product and the first new inhaled mechanism drug for the treatment of COPD in more than 20 years. Analysts predict that by the mid-2030s, the drug's annual sales could reach nearly $4 billion.
Chronic obstructive pulmonary disease (COPD) is a serious chronic respiratory disease. As of 2019, an estimated 390 million people worldwide suffer from COPD, making it the fourth leading cause of death worldwide. There is currently no cure for COPD.
Ensifentrine (Ohtuvayre) is a world-first phosphodiesterase 3/4 (PDE3/4) inhibitor developed by Verona Pharma, which has both bronchodilator and non-steroidal anti-inflammatory effects. In use, the product enters the lungs directly through a standard jet nebulizer, without the need for high inhalation airflow or complex hand-exhalation coordination.
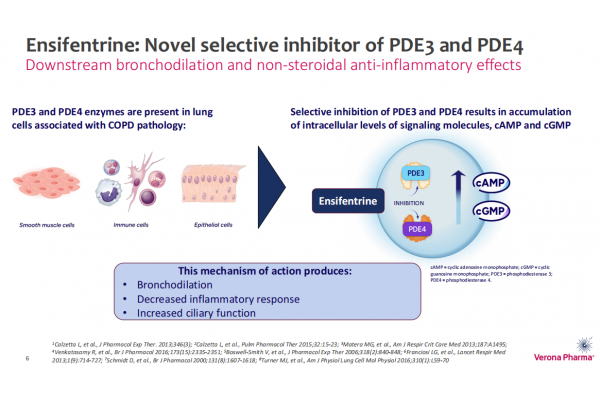
In June 2024, the drug was approved by the U.S. FDA for marketing as a maintenance therapy for adult patients with chronic obstructive pulmonary disease (COPD). The FDA's approval was based on data from two Phase III clinical studies, ENHANCE-1 and ENHANCE-2, in which Ensifentrine showed statistically significant and clinically meaningful improvements in lung function.
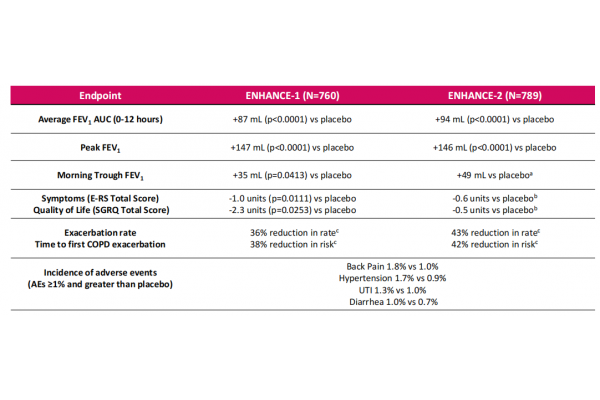
Thanks to its new mechanism and wide applicability in COPD patients, Ensifentrine's sales have grown rapidly since its launch, with cumulative sales exceeding $100 million in 8 months, and sales reaching $71.3 million in Q1 2025 alone. With Merck's entry, Ohtuvayre is expected to benefit more COPD patients and help the drug quickly increase in sales and become a blockbuster drug.
Verona Pharma is also exploring ensifentrine for the treatment of non-cystic fibrosis bronchiectasis, cystic fibrosis and asthma. Verona Pharma is also developing ensifentrine + LAMA (Nebulizer) fixed-dose combination therapy, as well as ensifentrine inhalation powder (DPI) and metered dose inhaler (MDI).
It is worth mentioning that Nuance Pharma has the exclusive rights to clinical development and commercialization of Ensifentrine in Greater China. Currently, the Phase III COPD clinical trial ENHANCE-CHINA conducted in China has achieved positive top-line results, and Nuance Pharma plans to submit a new drug application to NMPA in the second half of 2025.
In addition, according to Yaozhi data, there are also many domestic pharmaceutical companies that are developing PDE3/4 inhibitors. Many of them, such as TQC3721 of Sinopharm Tianqing, HSK39004 of Hisun Pharmaceutical and HRS-9821 of Hengrui Medicine, have entered the clinical stage. Among them, TQC3721 suspension of Sinopharm Tianqing has been approved by CDE to conduct Phase III registered clinical research for the maintenance treatment of COPD.
Source:https://news.yaozh.com/archive/45759.html
By editorRead more on
- Rovaxitinib approved for marketing, filling the demand for myelofibrosis treatment March 2, 2026
- Warrant Pharmaceuticals’ active pharmaceutical ingredient receives Brazil’s first official GMP certification March 2, 2026
- Merck’s New Story March 2, 2026
- Rongchang Biotechnology has turned a profit! March 2, 2026
- Jiuyuan Gene’s “Simeglucopyranoside” for weight loss (Jikeqin®) has been submitted for market approval March 2, 2026
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



