FDA Approval for Eversense – Continuous Glucose Monitoring System
June 27, 2018
Source: MobiHealthNews
 1,162
1,162
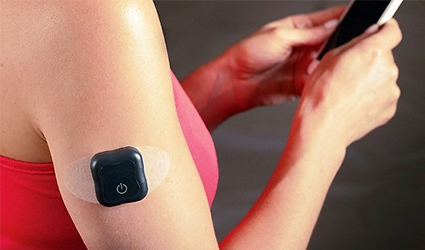
The FDA has finally approved to market the implantable continuous glucose monitoring system named Eversense, from Senseonics, in the United States.
Dr. Scott Gottlieb, the FDA Commissioner said, “The FDA is committed to advancing novel products that leverage digital technology to improve patient care; these technologies allow patients to gain better control over their health. This approval of a more seamless digital system that gives patients the ability to effectively manage a chronic disease like diabetes is a vivid illustration of the potential for these mobile platforms.”
Senseonics has been waiting for the go-ahead from the FDA since November 2016. Tim Goodnow, President and CEO of Senseonics said, “We actually have been commercializing the product for about 21 months in Europe, so we’ve got some experience with our manufacturing, our quality systems, and we do that predominately through our partner Roche, and now the US is our largest market. It’s about 75 to 80 percent of the global glucose monitoring market opportunity for CGM, and we’re happy to be here.”
Approval from the FDA is for the Eversense model which covers the 90-day monitoring model, whereas the available version in Europe lasts for 180 days.
Goodnow further added, “They requested that because they had never approved an implantable product before for glucose monitoring. So [they held the panel] to make sure that they asked all the right questions and that they had representatives from the medical community to buy-in on their decisions, and I think that really empowered the FDA to feel strongly about moving quickly to that final approval.”
Goodnow confirmed that Eversense will be available on the market by the end of July 2018.
By DduRead more on
- Bayer’s Kerendia approved in the US to slow CKD in type 2 diabetes patients August 26, 2021
- Autoantibody order, timing predict genetically at-risk children most likely to get type 1 diabetes October 30, 2020
- Machine Learning Applied to Manage Treatment-Resistant Depression September 3, 2018
- Merck’s New Digital Diabetes Coaching to Educate and Manage Patients August 31, 2018
- UK Advertising Regulator Bans Natural Cycles’ Facebook Ad August 31, 2018
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



