Data Byte
November 27, 2020
Source: drugdu
 630
630
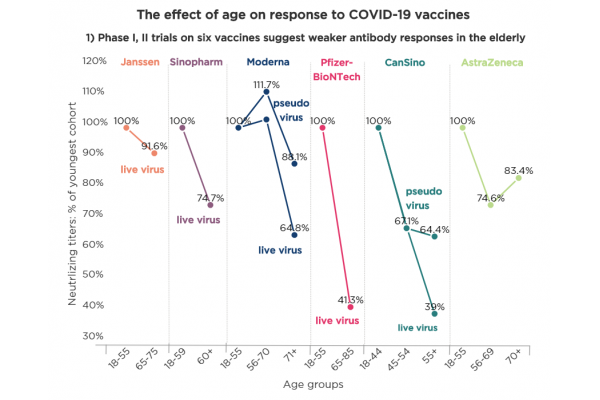
A COMPARISON OF NEUTRALIZATION TITERS AND PROTECTION DATA ACROSS CANDIDATES AND AGE GROUPS
A pressing question about COVID-19 vaccines is how well they will perform in the elderly, who are at higher risk for severe disease. Wednesday’s report of strong efficacy in subjects over 65 given Pfizer and BioNTech’s mRNA vaccine could portend good news for several of the next candidates.
In early-stage clinical trials, at least four other vaccines produced neutralizing antibody levels in non-elderly adults that were in line with those of BNT162b2 from Pfizer Inc. (NYSE:PFE) and BioNTech SE (NASDAQ:BNTX), and for the most part, the vaccines’ titers fell less with age than BNT162b2.
Only Ad5-nCoV from CanSino Biologics Inc. (HKEX:6185; Shanghai:688185) produced somewhat lower neutralization titers that fell farther with age than BNT162b2 in a live virus assay.
Moderna Inc. (NASDAQ:MRNA) announced Monday that mRNA-1273 yielded a 94.5% protection rate at an interim analysis of a Phase III trial; the data were not broken down by age. Additional Phase III data are expected shortly.
AstraZeneca plc (LSE:AZN; NASDAQ:AZN) reported Thursday neutralization titers across age groups from a Phase II trial of AZD1222; the company expects Phase III data by year-end, if not sooner.
From: https://www.biocentury.com/article/632098/the-first-covid-19-vaccine-data-in-the-elderly-may-bode-well-for-the-next-candidates-data-byte?tag=cov19count&return_feed=%2Fcoronavirus
By editorRead more on
- Positive results for Pfizer/BioNTech’s COVID-19 vaccine in 12-15 year olds April 2, 2021
- AZ’s vaccine safety review shows ‘no evidence’ of an increased risk of blood clots March 16, 2021
- New social media campaign launched to support COVID-19 vaccine roll-out in the UK February 23, 2021
- NHS has offered COVID-19 vaccine to all eligible care homes in England February 2, 2021
- UK medicines regulator gives approval for first UK COVID-19 vaccine December 3, 2020
your submission has already been received.
OK
Subscribe
Please enter a valid Email address!
Submit
The most relevant industry news & insight will be sent to you every two weeks.



